A novel duodenal-release formulation of caraway oil and L-menthol is a safe, effective and well tolerated therapy for functional dyspepsia
- PMID: 35255832
- PMCID: PMC8900420
- DOI: 10.1186/s12876-022-02181-5
A novel duodenal-release formulation of caraway oil and L-menthol is a safe, effective and well tolerated therapy for functional dyspepsia
Abstract
Background: A randomized, placebo-controlled clinical trial (FDREST) of a novel formulation of caraway oil and L-menthol (COLM-SST) demonstrated symptom relief in patients with functional dyspepsia (FD). Two follow-up studies were conducted to evaluate patient satisfaction, self-regulated dosing, and long-term safety data: FDACT, Functional Dyspepsia Adherence and Compliance Trial, and FDSU36, Functional Dyspepsia Safety Update at 36 months.
Methods: A patient reported outcomes (PRO) questionnaire was designed and distributed online to assess real-world satisfaction and dosing frequency of open-label COLM-SST in patients with FD. A separate study analyzing voluntary safety surveillance data evaluated the frequency and severity of reported adverse events (AEs).
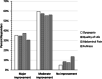
Results: A total of 600 FD patients were enrolled in the PRO study. Ninety five percent of respondents reported a major or moderate improvement in their FD symptoms and 91.7% indicated a major or moderate improvement in quality of life (QOL) using COLM-SST. Between 1 and 4 capsules were consumed daily by 91.2% of respondents, with 56.2% taking them before meals. Symptom relief was rapid, with 86.4% of respondents indicating relief within 2 h of taking COLM-SST. Few adverse events (AEs) were reported (0.0187%) by patients using COLM-SST. No serious AEs were identified.
Conclusion: COLM-SST is safe, well tolerated, and provides rapid relief of FD symptoms. These findings, demonstrated in the FDREST trial, were further supported by a large prospective PRO study evaluating self-regulated dosing frequency, symptom improvement, and QOL. COLM-SST was well-tolerated based on review of AE data at 36 months.
Keywords: Abdominal pain; Caraway oil; Dyspepsia; Functional dyspepsia; L-menthol.
© 2022. The Author(s).
Conflict of interest statement
BEL: Scientific advisory boards for Allergan, Salix, IM Health Science, Ironwood, Arena and is a consultant for Viver, Nestlé Health Science; WDC: Board member—American College of Gastroenterology, GI on Demand, International Foundation of Functional GI Disorders, Rome Foundation; Consultant—AbbVie, Alfasigma, Allakos, Alnylam, Arena, Bayer, Biomerica, Cosmo, IM Health Science, Ironwood, QOL Medical, Nestlé Health Science, Phathom, Redhill, Ritter, Salix/Valeant, Takeda, Urovant, Vibrant; Grant/Research Support: Bioamerica, Commonwealth Diagnostics International, QOL Medical, Salix; Stock/Stock options: GI on Demand, Modify Health; MSE: Consultant to Nestlé Health Science; Speaker for BMS, Redhill, Gilead, Salix. Pfizer. SMS: Consultant to Nestlé Health Science; PC: none; LRZ: employee Nestlé Health Science; BDC: Consultant to Nestlé Health Science.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical