Cyanidin 3-O-arabinoside suppresses DHT-induced dermal papilla cell senescence by modulating p38-dependent ER-mitochondria contacts
- PMID: 35255899
- PMCID: PMC8900350
- DOI: 10.1186/s12929-022-00800-7
Cyanidin 3-O-arabinoside suppresses DHT-induced dermal papilla cell senescence by modulating p38-dependent ER-mitochondria contacts
Abstract
Background: Androgenetic alopecia (AGA) is a genetic disorder caused by dihydrotestosterone (DHT), accompanied by the senescence of androgen-sensitive dermal papilla cells (DPCs) located in the base of hair follicles. DHT causes DPC senescence in AGA through mitochondrial dysfunction. However, the mechanism of this pathogenesis remains unknown. In this study, we investigated the protective role of cyanidins on DHT-induced mitochondrial dysfunction and DPC senescence and the regulatory mechanism involved.
Methods: DPCs were used to investigate the effect of DHT on mitochondrial dysfunction with MitoSOX and Rhod-2 staining. Senescence-associated β-galactosidase activity assay was performed to examine the involvement of membrane AR-mediated signaling in DHT-induced DPC senescence. AGA mice model was used to study the cyanidins on DHT-induced hair growth deceleration.
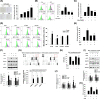
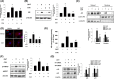
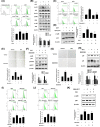
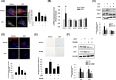
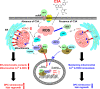
Results: Cyanidin 3-O-arabinoside (C3A) effectively decreased DHT-induced mtROS accumulation in DPCs, and C3A reversed the DHT-induced DPC senescence. Excessive mitochondrial calcium accumulation was blocked by C3A. C3A inhibited p38-mediated voltage-dependent anion channel 1 (VDAC1) expression that contributes to mitochondria-associated ER membrane (MAM) formation and transfer of calcium via VDAC1-IP3R1 interactions. DHT-induced MAM formation resulted in increase of DPC senescence. In AGA mice models, C3A restored DHT-induced hair growth deceleration, which activated hair follicle stem cell proliferation.
Conclusions: C3A is a promising natural compound for AGA treatments against DHT-induced DPC senescence through reduction of MAM formation and mitochondrial dysfunction.
Keywords: Androgen receptor; Cyanidin 3-O-arabinoside; DHT; Mitochondrial calcium; Senescence.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Kaufman KD. Androgens and alopecia. Mol Cell Endocrinol. 2002;198(1–2):89–95. - PubMed
-
- Premanand A, Reena RB. Androgen modulation of Wnt/β-catenin signaling in androgenetic alopecia. Arch Dermatol Res. 2018;310(5):391–399. - PubMed
-
- Kwack MH, Sung YK, Chung EJ, Im SU, Ahn JS, Kim MK, et al. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J Invest Dermatol. 2008;128(2):262–269. - PubMed
-
- Huang WY, Huang YC, Huang KS, Chan CC, Chiu HY, Tsai RY, et al. Stress-induced premature senescence of dermal papilla cells compromises hair follicle epithelial-mesenchymal interaction. J Dermatol Sci. 2017;86(2):114–122. - PubMed
-
- Bahta AW, Farjo N, Farjo B, Philpott MP. Premature senescence of balding dermal papilla cells in vitro is associated with p16(INK4a) expression. J Invest Dermatol. 2008;128(5):1088–1094. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

