Immunotherapy combining tumor and endothelium cell lysis with immune enforcement by recombinant MIP-3α Newcastle disease virus in a vessel-targeting liposome enhances antitumor immunity
- PMID: 35256516
- PMCID: PMC8905871
- DOI: 10.1136/jitc-2021-003950
Immunotherapy combining tumor and endothelium cell lysis with immune enforcement by recombinant MIP-3α Newcastle disease virus in a vessel-targeting liposome enhances antitumor immunity
Abstract
Background: Several agents for oncolytic immunotherapy have been approved for clinical use, but monotherapy is modest for most oncolytic agents. The combination of several therapeutic strategies through recombinant and nanotechnology to engineer multifunctional oncolytic viruses for oncolytic immunotherapy is a promising strategy.
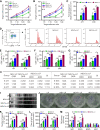
Methods: An endothelium-targeting iRGD-liposome encapsulating a recombinant Newcastle disease virus (NDV), which expresses the dendritic cell (DC) chemokine MIP-3α (iNDV3α-LP), and three control liposomes were constructed. MIP-3α, HMGB1, IgG, and ATP were detected by western blotting or ELISA. The chemotaxis of DCs was examined by Transwell chambers. The phenotypes of the immune cells were analyzed by flow cytometry. The antitumor efficiency was investigated in B16 and 4T1 tumor-bearing mice. Immunofluorescence and immunohistochemistry were used to observe the localization of liposomes, molecular expression and angiogenesis. Synergistic index was calculated using the data of tumor volume, tumor angiogenesis and tumor-infiltrating lymphocytes.
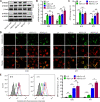
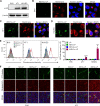
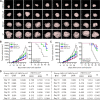
Results: Compared with NDV-LP, treatment with iNDV3α-LP and NDV3α-LP induced stronger virus replication and cell lysis in B16 and 4T1 tumor cells and human umbilical vein endothelial cells (HUVECs) with the best response observed following iNDV3α-LP treatment. B16 and 4T1 cells treated with iNDV3α-LP produced more damage-associated molecular pattern molecules, including secreted HMGB1, ATP, and calreticulin. Moreover, iNDV3α-LP specifically bound to αvβ3-expressing 4T1 cells and HUVECs and to tumor neovasculature. Tumor growth was significantly suppressed, and survival was longer in iNDV3α-LP-treated B16-bearing and 4T1-bearing mice. A mechanism study showed that iNDV3α-LP treatment initiated the strongest tumor-specific cellular and humoral immune response. Moreover, iNDV3α-LP treatment could significantly suppress tumor angiogenesis and reverse the tumor immune suppressive microenvironment in both B16-bearing and 4T1-bearing mice.
Conclusions: In this study, iNDV3α-LP had several functions, such as tumor and vessel lysis, MIP-3α immunotherapy, and binding to αvβ3-expressing tumor and its neovasculature. iNDV3α-LP treatment significantly suppressed tumor angiogenesis and reversed the tumor immunosuppressive microenvironment. These findings offer a strong rationale for further clinical investigation into a combination strategy for oncolytic immunotherapy, such as the formulation iNDV3α-LP in this study.
Keywords: combined modality therapy; immunogenicity, vaccine; immunotherapy; oncolytic virotherapy; tumor microenvironment.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



Similar articles
-
A recombinant oncolytic Newcastle virus expressing MIP-3α promotes systemic antitumor immunity.J Immunother Cancer. 2020 Aug;8(2):e000330. doi: 10.1136/jitc-2019-000330. J Immunother Cancer. 2020. PMID: 32759233 Free PMC article.
-
Synergistic Anti-Tumor Effects of Newcastle Disease Virus and Doxorubicin: Evidence from A Murine Breast Cancer Model.Int Immunopharmacol. 2024 Dec 25;143(Pt 2):113481. doi: 10.1016/j.intimp.2024.113481. Epub 2024 Oct 29. Int Immunopharmacol. 2024. PMID: 39467343
-
Evaluation of the oncolytic property of recombinant Newcastle disease virus strain R2B in 4T1 and B16-F10 cells in-vitro.Res Vet Sci. 2021 Oct;139:159-165. doi: 10.1016/j.rvsc.2021.07.028. Epub 2021 Jul 25. Res Vet Sci. 2021. PMID: 34332418
-
Counteracting Immunosuppression in the Tumor Microenvironment by Oncolytic Newcastle Disease Virus and Cellular Immunotherapy.Int J Mol Sci. 2022 Oct 27;23(21):13050. doi: 10.3390/ijms232113050. Int J Mol Sci. 2022. PMID: 36361831 Free PMC article. Review.
-
Newcastle disease virus: a promising agent for tumour immunotherapy.Clin Exp Pharmacol Physiol. 2012 Aug;39(8):725-30. doi: 10.1111/j.1440-1681.2011.05662.x. Clin Exp Pharmacol Physiol. 2012. PMID: 22211810 Review.
Cited by
-
Progesterone modulates the immune microenvironment to suppress ovalbumin-induced airway inflammation by inhibiting NETosis.Sci Rep. 2024 Jul 26;14(1):17241. doi: 10.1038/s41598-024-66439-6. Sci Rep. 2024. PMID: 39060348 Free PMC article.
-
Nanoengineering-armed oncolytic viruses drive antitumor response: progress and challenges.MedComm (2020). 2024 Oct 10;5(10):e755. doi: 10.1002/mco2.755. eCollection 2024 Oct. MedComm (2020). 2024. PMID: 39399642 Free PMC article. Review.
-
Influence of Elasticity of Hydrogel Nanoparticles on Their Tumor Delivery.Adv Sci (Weinh). 2022 Oct;9(29):e2202644. doi: 10.1002/advs.202202644. Epub 2022 Aug 18. Adv Sci (Weinh). 2022. PMID: 35981891 Free PMC article.
-
Oncolytic Viruses: Immunotherapy Drugs for Gastrointestinal Malignant Tumors.Front Cell Infect Microbiol. 2022 Jun 3;12:921534. doi: 10.3389/fcimb.2022.921534. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35719333 Free PMC article. Review.
-
Harnessing Oncolytic Viruses for Targeted Therapy in Triple-Negative Breast Cancer.Int J Med Sci. 2025 Apr 13;22(9):2186-2207. doi: 10.7150/ijms.105683. eCollection 2025. Int J Med Sci. 2025. PMID: 40303488 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
