Differential Activation of Pain Circuitry Neuron Populations in a Mouse Model of Spinal Cord Injury-Induced Neuropathic Pain
- PMID: 35256528
- PMCID: PMC8994550
- DOI: 10.1523/JNEUROSCI.1596-21.2022
Differential Activation of Pain Circuitry Neuron Populations in a Mouse Model of Spinal Cord Injury-Induced Neuropathic Pain
Abstract
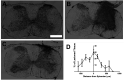
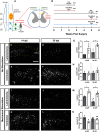
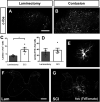
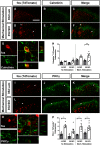
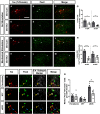
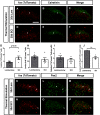
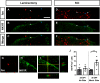
Neuropathic pain (NP) is one of the most common and debilitating comorbidities of spinal cord injury (SCI). Current therapies are often ineffective due in part to an incomplete understanding of underlying pathogenic mechanisms. In particular, it remains unclear how SCI leads to dysfunction in the excitability of nociceptive circuitry. The immediate early gene c-Fos has long been used in pain processing locations as a marker of neuronal activation. We employed a mouse reporter line with fos-promoter driven Cre-recombinase to define neuronal activity changes in relevant pain circuitry locations following cervical spinal cord level (C)5/6 contusion (using both females and males), a SCI model that results in multiple forms of persistent NP-related behavior. SCI significantly increased activation of cervical dorsal horn (DH) projection neurons, as well as induced a selective reduction in the activation of a specific DH projection neuron subpopulation that innervates the periaqueductal gray (PAG), an important brain region involved in descending inhibitory modulation of DH pain transmission. SCI also increased the activation of both protein kinase C (PKC)γ and calretinin excitatory DH interneuron populations. Interestingly, SCI promoted a significant decrease in the activation selectively of neuronal nitric oxide synthase (nNOS)-expressing inhibitory interneurons of cervical DH. In addition, SCI altered activation of various supraspinal neuron populations associated with pain processing, including a large increase in thalamus and a significant decrease in PAG. These findings reveal a complex and diverse set of SCI-induced neuron activity changes across the pain circuitry neuraxis. Moving forward, these results can be used to inform therapeutic targeting of defined neuronal populations in NP.SIGNIFICANCE STATEMENT Neuropathic pain (NP) is one of the most common and highly debilitating comorbidities of spinal cord injury (SCI). Unfortunately, current therapies are often ineffective due in part to an incomplete understanding of underlying pathogenic mechanisms. In particular, it remains unclear how SCI leads to dysfunction in excitability of nociceptive circuitry. Using a FosTRAP2 reporter mouse line in a model of SCI-induced NP, we show SCI alters activation of a number of important interneuron and projection neuron populations across relevant spinal cord and brain locations of the pain circuitry neuraxis. These data suggest a role for maladaptive plasticity involving specific subpopulations of neurons and circuits in driving SCI-induced chronic pain. Moving forward, these results can be used to inform therapeutic targeting of defined neuronal populations in NP.
Keywords: TRAP; dorsal horn; fos; interneuron; neuropathic pain; spinal cord injury.
Copyright © 2022 the authors.
Figures
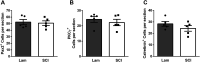

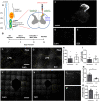
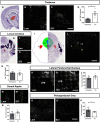
References
-
- Allen Institute (2004) Allen mouse brain atlas. Available at https://mouse.brain-map.org/static/atlas.
-
- Bernardi PS, Valtschanoff JG, Weinberg RJ, Schmidt HH, Rustioni A (1995) Synaptic interactions between primary afferent terminals and GABA and nitric oxide-synthesizing neurons in superficial laminae of the rat spinal cord. J Neurosci 15:1363–1371. 10.1523/JNEUROSCI.15-02-01363.1995 - DOI - PMC - PubMed
-
- Brown EV, Falnikar A, Heinsinger N, Cheng L, Andrews CE, DeMarco M, Lepore AC (2021) Cervical spinal cord injury-induced neuropathic pain in male mice is associated with a persistent pro-inflammatory macrophage/microglial response in the superficial dorsal horn. Exp Neurol 343:113757. 10.1016/j.expneurol.2021.113757 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
