Interleukin 13 on Microglia is Neurotoxic in Lipopolysaccharide-injected Striatum in vivo
- PMID: 35256543
- PMCID: PMC8907255
- DOI: 10.5607/en21032
Interleukin 13 on Microglia is Neurotoxic in Lipopolysaccharide-injected Striatum in vivo
Abstract
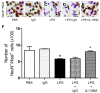
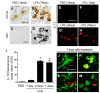
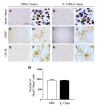
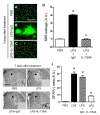
To explore the potential function of interleukin-13 (IL-13), lipopolysaccharide (LPS) or PBS as a control was unilaterally microinjected into striatum of rat brain. Seven days after LPS injection, there was a significant loss of neurons and microglial activation in the striatum, visualized by immunohistochemical staining against neuronal nuclei (NeuN) and the OX-42 (complement receptor type 3, CR3), respectively. In parallel, IL-13 immunoreactivity was increased as early as 3 days and sustained up to 7 days post LPS injection, compared to PBS-injected control and detected exclusively within microglia. Moreover, GFAP immunostaining and blood brain barrier (BBB) permeability evaluation showed the loss of astrocytes and disruption of BBB, respectively. By contrast, treatment with IL-13 neutralizing antibody (IL-13NA) protects NeuN+ neurons against LPS-induced neurotoxicity in vivo . Accompanying neuroprotection, IL-13NA reduced loss of GFAP+ astrocytes and damage of BBB in LPS-injected striatum. Intriguingly, treatment with IL-13NA produced neurotrophic factors (NTFs) on survived astrocytes in LPS-injected rat striatum. Taken together, the present study suggests that LPS induces expression of IL-13 on microglia, which contributes to neurodegeneration via damage on astrocytes and BBB disruption in the striatum in vivo.
Keywords: Astrocytes; Interleukin 13; Microglia; Neurodegeneration; Neurotrophic factor; Striatum.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
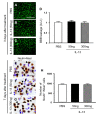

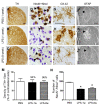
Similar articles
-
Interleukin-4 Aggravates LPS-Induced Striatal Neurodegeneration In Vivo via Oxidative Stress and Polarization of Microglia/Macrophages.Int J Mol Sci. 2022 Jan 5;23(1):571. doi: 10.3390/ijms23010571. Int J Mol Sci. 2022. PMID: 35008995 Free PMC article.
-
Microglia expressing interleukin-13 undergo cell death and contribute to neuronal survival in vivo.Glia. 2004 Apr 15;46(2):142-52. doi: 10.1002/glia.10357. Glia. 2004. PMID: 15042582
-
Interleukin-4 Contributes to Degeneration of Dopamine Neurons in the Lipopolysaccharide-treated Substantia Nigra in vivo.Exp Neurobiol. 2018 Aug;27(4):309-319. doi: 10.5607/en.2018.27.4.309. Epub 2018 Aug 30. Exp Neurobiol. 2018. PMID: 30181693 Free PMC article.
-
Interleukin-10 Protection against Lipopolysaccharide-Induced Neuro-Inflammation and Neurotoxicity in Ventral Mesencephalic Cultures.Int J Mol Sci. 2015 Dec 28;17(1):25. doi: 10.3390/ijms17010025. Int J Mol Sci. 2015. PMID: 26729090 Free PMC article.
-
Mesenchymal stem cells stabilize the blood-brain barrier through regulation of astrocytes.Stem Cell Res Ther. 2015 Sep 29;6:187. doi: 10.1186/s13287-015-0180-4. Stem Cell Res Ther. 2015. PMID: 26420371 Free PMC article.
Cited by
-
Neonatal Chlamydia muridarum respiratory infection causes neuroinflammation within the brainstem during the early postnatal period.J Neuroinflammation. 2024 Jun 15;21(1):158. doi: 10.1186/s12974-024-03150-3. J Neuroinflammation. 2024. PMID: 38879567 Free PMC article.
-
Effects of Antiretroviral Treatment on Central and Peripheral Immune Response in Mice with EcoHIV Infection.Cells. 2024 May 20;13(10):882. doi: 10.3390/cells13100882. Cells. 2024. PMID: 38786105 Free PMC article.
-
The inflammatory injury in the striatal microglia-dopaminergic-neuron crosstalk involved in Tourette syndrome development.Front Immunol. 2023 Apr 28;14:1178113. doi: 10.3389/fimmu.2023.1178113. eCollection 2023. Front Immunol. 2023. PMID: 37187752 Free PMC article.
References
-
- Nam JH, Park ES, Won SY, Lee YA, Kim KI, Jeong JY, Baek JY, Cho EJ, Jin M, Chung YC, Lee BD, Kim SH, Kim EG, Byun K, Lee B, Woo DH, Lee CJ, Kim SR, Bok E, Kim YS, Ahn TB, Ko HW, Brahmachari S, Pletinkova O, Troconso JC, Dawson VL, Dawson TM, Jin BK. TRPV1 on astrocytes rescues nigral dopamine neurons in Parkinson's disease via CNTF. Brain. 2015;138(Pt 12):3610–3622. doi: 10.1093/brain/awv297. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

