Bordetella pertussis-infected innate immune cells drive the anti-pertussis response of human airway epithelium
- PMID: 35256671
- PMCID: PMC8901624
- DOI: 10.1038/s41598-022-07603-8
Bordetella pertussis-infected innate immune cells drive the anti-pertussis response of human airway epithelium
Abstract
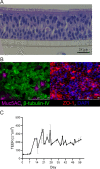
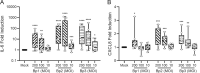
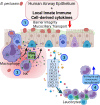
Pertussis is a severe respiratory tract infection caused by Bordetella pertussis. This bacterium infects the ciliated epithelium of the human airways. We investigated the epithelial cell response to B. pertussis infection in primary human airway epithelium (HAE) differentiated at air-liquid interface. Infection of the HAE cells mimicked several hallmarks of B. pertussis infection such as reduced epithelial barrier integrity and abrogation of mucociliary transport. Our data suggests mild immunological activation of HAE by B. pertussis indicated by secretion of IL-6 and CXCL8 and the enrichment of genes involved in bacterial recognition and innate immune processes. We identified IL-1β and IFNγ, present in conditioned media derived from B. pertussis-infected macrophage and NK cells, as essential immunological factors for inducing robust chemokine secretion by HAE in response to B. pertussis. In transwell migration assays, the chemokine-containing supernatants derived from this HAE induced monocyte migration. Our data suggests that the airway epithelium on its own has a limited immunological response to B. pertussis and that for a broad immune response communication with local innate immune cells is necessary. This highlights the importance of intercellular communication in the defense against B. pertussis infection and may assist in the rational design of improved pertussis vaccines.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

