High burden of clonal hematopoiesis in first responders exposed to the World Trade Center disaster
- PMID: 35256801
- PMCID: PMC9394171
- DOI: 10.1038/s41591-022-01708-3
High burden of clonal hematopoiesis in first responders exposed to the World Trade Center disaster
Abstract
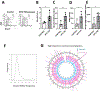
The terrorist attacks on the World Trade Center (WTC) created an unprecedented environmental exposure to aerosolized dust, gases and potential carcinogens. Clonal hematopoiesis (CH) is defined as the acquisition of somatic mutations in blood cells and is associated with smoking and exposure to genotoxic stimuli. Here we show that deep targeted sequencing of blood samples identified a significantly higher proportion of WTC-exposed first responders with CH (10%; 48 out of 481) when compared with non-WTC-exposed firefighters (6.7%; 17 out of 255; odds ratio, 3.14; 95% confidence interval, 1.64-6.03; P = 0.0006) after controlling for age, sex and race/ethnicity. The frequency of somatic mutations in WTC-exposed first responders showed an age-related increase and predominantly affected DNMT3A, TET2 and other CH-associated genes. Exposure of lymphoblastoid cells to WTC particulate matter led to dysregulation of DNA replication at common fragile sites in vitro. Moreover, mice treated with WTC particulate matter developed an increased burden of mutations in hematopoietic stem and progenitor cell compartments. In summary, the high burden of CH in WTC-exposed first responders provides a rationale for enhanced screening and preventative efforts in this population.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures









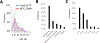
References
Publication types
MeSH terms
Substances
Grants and funding
- S10 OD025092/OD/NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- U01 OH011300/OH/NIOSH CDC HHS/United States
- U19 HL065962/HL/NHLBI NIH HHS/United States
- F30 DK127699/DK/NIDDK NIH HHS/United States
- U01OH011933/ACL/ACL HHS/United States
- P50 GM115305/GM/NIGMS NIH HHS/United States
- R01 HD074711/HD/NICHD NIH HHS/United States
- U01 OH011855/OH/NIOSH CDC HHS/United States
- S10 OD017985/OD/NIH HHS/United States
- T32 GM152284/GM/NIGMS NIH HHS/United States
- S10 RR025141/RR/NCRR NIH HHS/United States
- U01 OH012069/OH/NIOSH CDC HHS/United States
- RC2 GM092618/GM/NIGMS NIH HHS/United States
- U01 HG004798/HG/NHGRI NIH HHS/United States
- R00 HL136870/HL/NHLBI NIH HHS/United States
- R01 NS032830/NS/NINDS NIH HHS/United States
- T32 GM007347/GM/NIGMS NIH HHS/United States
- U01 HG006378/HG/NHGRI NIH HHS/United States
- U01 OH012271/OH/NIOSH CDC HHS/United States
- U01 OH011933/OH/NIOSH CDC HHS/United States
- UL1 TR002243/TR/NCATS NIH HHS/United States
- UL1 TR000445/TR/NCATS NIH HHS/United States
- R01 HL119326/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

