Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation
- PMID: 35256954
- PMCID: PMC8897038
- DOI: 10.1016/j.apsb.2021.08.016
Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation
Abstract
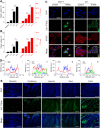
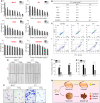
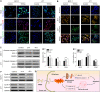
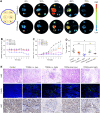
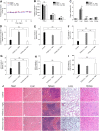
Although several artificial nanotherapeutics have been approved for practical treatment of metastatic breast cancer, their inefficient therapeutic outcomes, serious adverse effects, and high cost of mass production remain crucial challenges. Herein, we developed an alternative strategy to specifically trigger apoptosis of breast tumors and inhibit their lung metastasis by using natural nanovehicles from tea flowers (TFENs). These nanovehicles had desirable particle sizes (131 nm), exosome-like morphology, and negative zeta potentials. Furthermore, TFENs were found to contain large amounts of polyphenols, flavonoids, functional proteins, and lipids. Cell experiments revealed that TFENs showed strong cytotoxicities against cancer cells due to the stimulation of reactive oxygen species (ROS) amplification. The increased intracellular ROS amounts could not only trigger mitochondrial damage, but also arrest cell cycle, resulting in the in vitro anti-proliferation, anti-migration, and anti-invasion activities against breast cancer cells. Further mice investigations demonstrated that TFENs after intravenous (i.v.) injection or oral administration could accumulate in breast tumors and lung metastatic sites, inhibit the growth and metastasis of breast cancer, and modulate gut microbiota. This study brings new insights to the green production of natural exosome-like nanoplatform for the inhibition of breast cancer and its lung metastasis via i.v. and oral routes.
Keywords: AF633, Alexa Fluor 633-labeled phalloidin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, urea nitrogen; Breast cancer; CDK, CYCLIN-dependent kinase; CRE, creatinine; DAF-FM DA, 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate; DAPI, 4′,6-diamidino-2-phenylindole; DCFH-DA, dichloro-dihydro-fluorescein diacetate; DGDG, digalactosyl diacylglycerols; DHE, dihydroethidium; DLS, dynamic light scattering; DiO, 3,3′-dioctadecyloxacarbocyanine perchlorate; DiR, 1,1′-dioctadecyl-3,3,3′′,3′-tetramethylindotricarbocyanine iodide; EC, epicatechin; ECG, epicatechin gallate; EGCG, epigallocatechin gallate; Exosome-like nanoparticle; FBS, fetal bovine serum; GIT, gastrointestinal tract; H&E, Hematoxylin & Eosin; HPLC, high-performance liquid chromatography; Intravenous injection; LC‒MS, liquid chromatography‒mass spectrometry; MFI, mean fluorescence intensity; MGDG, monogalactosyl diacylglycerols; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Metastasis; Microbiota modulation; NO, nitrogen monoxide; NPs, nanoparticles; OUT, operational taxonomic unit; Oral administration; PA, phosphatidic acids; PBS, phosphate-buffered saline; PC, phosphatidylcholines; PDI, polydispersity index; PE, phosphatidylethanolamines; PG, phosphatidylglycerol; PI, phosphatidylinositol; PLT, platelets; PMe, phosphatidylmethanol; PS, phosphatidylserine; RBC, red blood cell; RNS, reactive nitrogen species; ROS generation; ROS, reactive oxygen species; SA, superoxide anion; SQDG, sulphoquinovosyl diylyceride; TEM, transmission electron microscopy; TFENs, exosome-like NPs from tea flowers; TG, triglyceride; TUNEL, TdT-mediated dUTP Nick-end labeling; Tea flower; WBC, white blood cell.
© 2022 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures




References
-
- Tang Y., Wang Y., Kiani M.F., Wang B. Classification, treatment strategy, and associated drug resistance in breast cancer. Clin Breast Cancer. 2016;16:335–343. - PubMed
-
- Xiao Y., Cong M., Li J., He D., Wu Q., Tian P., et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell. 2021;39:423–437 e7. - PubMed
-
- Poonia N., Lather V., Pandita D. Mesoporous silica nanoparticles: a smart nanosystem for management of breast cancer. Drug Discov Today. 2018;23:315–332. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

