H2 Inhibits the Formation of Neutrophil Extracellular Traps
- PMID: 35257042
- PMCID: PMC8897170
- DOI: 10.1016/j.jacbts.2021.11.005
H2 Inhibits the Formation of Neutrophil Extracellular Traps
Abstract
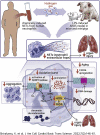
Neutrophil extracellular traps (NETs) contribute to inflammatory pathogenesis in numerous conditions, including infectious and cardiovascular diseases, and have attracted attention as potential therapeutic targets. H2 acts as an antioxidant and has been clinically and experimentally proven to ameliorate inflammation. This study was performed to investigate whether H2 could inhibit NET formation and excessive neutrophil activation. Neutrophils isolated from the blood of healthy volunteers were stimulated with phorbol-12-myristate-13-acetate (PMA) or the calcium ionophore A23187 in H2-exposed or control media. Compared with control neutrophils, PMA- or A23187-stimulated human neutrophils exposed to H2 exhibited reduced neutrophil aggregation, citrullination of histones, membrane disruption by chromatin complexes, and release of NET components. CXCR4high neutrophils are highly prone to NETs, and H2 suppressed Ser-139 phosphorylation in H2AX, a marker of DNA damage, thereby suppressing the induction of CXCR4 expression. H2 suppressed both myeloperoxidase chlorination activity and production of reactive oxygen species to the same degree as N-acetylcysteine and ascorbic acid, while showing a more potent ability to inhibit NET formation than these antioxidants do in PMA-stimulated neutrophils. Although A23187 formed NETs in a reactive oxygen species-independent manner, H2 inhibited A23187-induced NET formation, probably via direct inhibition of peptidyl arginine deiminase 4-mediated histone citrullination. Inhalation of H2 inhibited the formation and release of NET components in the blood and bronchoalveolar lavage fluid in animal models of lipopolysaccharide-induced sepsis (mice and aged mini pigs). Thus, H2 therapy can be a novel therapeutic strategy for NETs associated with excessive neutrophil activation.
Keywords: BAL, bronchoalveolar lavage; CVD, cardiovascular disease; CitH3, citrullinated histone H3; H2; HOCl, hypochlorous acid; LPS, lipopolysaccharide; MI, myocardial infarction; MPO, myeloperoxidase; NAC, N-acetyl-L-cysteine; NET, neutrophil extracellular trap; PA, pulmonary artery; PADI4, peptidyl arginine deiminase 4; PMA, phorbol-12-myristate-13-acetate; ROS, reactive oxygen species; dsDNA, double-stranded DNA; neutrophil extracellular traps; phorbol-12-myristate-13-acetate.
© 2022 The Authors.
Conflict of interest statement
This research was funded by Japanese Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (B) grant 18H02812 (2018-2020) and grants from Doctors Man Co, Ltd (to MS); and JSPS Grant-in Aid for Young Scientists grant JP18K15197 (2018–2020), Grant in-Aid for JSPS Fellows grant JP19J00583H (2018-2020). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. Drs Kobayashi and Sano have received advisory fees and research fees from Doctors Man Co, Ltd. Dr Sano has received advisory fees and research fees from Taiyo Nippon Sanso; is the registered inventor of the following patents jointly filed by Keio University and Taiyo Nippon Sanso: hydrogen mixed gas supply device for medical purposes (patent number: 5631524), medicinal composition for improving prognosis after restart of patient’s own heartbeat, and medicinal composition for improving and/or stabilizing circulatory dynamics after onset of hemorrhagic shock; and 3 other patents (whose names are translated into English): pharmaceutical compositions for reducing weight loss after organ harvesting (joint application with Keio University and Taiyo Nippon Sanso), method for generating organ preservation solution containing hydrogen and organ preservation solution containing hydrogen (joint application with Keio University and Doctors Man, application number PCT/JP2019/045790). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures





References
-
- Brinkmann V., Reichard U., Goosmann C., et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. - PubMed
-
- Grover S.P., Mackman N. Neutrophils, NETs, and immunothrombosis. Blood. 2018;132(13):1360–1361. - PubMed
-
- Song C., Li H., Li Y., et al. NETs promote ALI/ARDS inflammation by regulating alveolar macrophage polarization. Exp Cell Res. 2019;382(2):111486. - PubMed
-
- Stiel L., Mayeur-Rousse C., Helms J., Meziani F., Mauvieux L. First visualization of circulating neutrophil extracellular traps using cell fluorescence during human septic shock-induced disseminated intravascular coagulation. Thromb Res. 2019;183:153–158. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

