Interplay of Low-Density Lipoprotein Receptors, LRPs, and Lipoproteins in Pulmonary Hypertension
- PMID: 35257044
- PMCID: PMC8897182
- DOI: 10.1016/j.jacbts.2021.09.011
Interplay of Low-Density Lipoprotein Receptors, LRPs, and Lipoproteins in Pulmonary Hypertension
Abstract
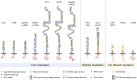
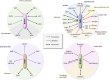
The low-density lipoprotein receptor (LDLR) gene family includes LDLR, very LDLR, and LDL receptor-related proteins (LRPs) such as LRP1, LRP1b (aka LRP-DIT), LRP2 (aka megalin), LRP4, and LRP5/6, and LRP8 (aka ApoER2). LDLR family members constitute a class of closely related multifunctional, transmembrane receptors, with diverse functions, from embryonic development to cancer, lipid metabolism, and cardiovascular homeostasis. While LDLR family members have been studied extensively in the systemic circulation in the context of atherosclerosis, their roles in pulmonary arterial hypertension (PAH) are understudied and largely unknown. Endothelial dysfunction, tissue infiltration of monocytes, and proliferation of pulmonary artery smooth muscle cells are hallmarks of PAH, leading to vascular remodeling, obliteration, increased pulmonary vascular resistance, heart failure, and death. LDLR family members are entangled with the aforementioned detrimental processes by controlling many pathways that are dysregulated in PAH; these include lipid metabolism and oxidation, but also platelet-derived growth factor, transforming growth factor β1, Wnt, apolipoprotein E, bone morpohogenetic proteins, and peroxisome proliferator-activated receptor gamma. In this paper, we discuss the current knowledge on LDLR family members in PAH. We also review mechanisms and drugs discovered in biological contexts and diseases other than PAH that are likely very relevant in the hypertensive pulmonary vasculature and the future care of patients with PAH or other chronic, progressive, debilitating cardiovascular diseases.
Keywords: ApoE, apolipoprotein E; Apoer2; BMP; BMPR, bone morphogenetic protein receptor; BMPR2; COPD, chronic obstructive pulmonary disease; CTGF, connective tissue growth factor; HDL, high-density lipoprotein; KO, knockout; LDL receptor related protein; LDL, low-density lipoprotein; LDLR; LDLR, low-density lipoprotein receptor; LRP; LRP, low-density lipoprotein receptor–related protein; LRP1; LRP1B; LRP2; LRP4; LRP5; LRP6; LRP8; MEgf7; Mesd, mesoderm development; PAH; PAH, pulmonary arterial hypertension; PASMC, pulmonary artery smooth muscle cell; PDGF; PDGFR-β, platelet-derived growth factor receptor-β; PH, pulmonary hypertension; PPARγ; PPARγ, peroxisome proliferator-activated receptor gamma; PVD; RV, right ventricle/ventricular; RVHF; RVSP, right ventricular systolic pressure; TGF-β1; TGF-β1, transforming growth factor β1; TGFBR, transforming growth factor β1 receptor; TNF, tumor necrosis factor receptor; VLDLR; VLDLR, very low density lipoprotein receptor; VSMC, vascular smooth muscle cell; Wnt; apolipoprotein E receptor 2; endothelial cell; gp330; low-density lipoprotein receptor; mRNA, messenger RNA; megalin; monocyte; multiple epidermal growth factor-like domains 7; pulmonary arterial hypertension; pulmonary vascular disease; right ventricle heart failure; smooth muscle cell; very low density lipoprotein receptor; β-catenin.
© 2022 The Authors.
Conflict of interest statement
Dr Herz was supported by grants from the National Heart, Lung, and Blood Institute (R37 HL063762), the National Institute on Aging (RF AG053391), the National Institute on Neurological Disorders and Stroke and National Institute on Aging (R01 NS093382), and BrightFocus (A2016396S); the Bluefield Project to Cure FTD; and a Harrington Scholar Innovator Award (2019). Dr Hansmann has received financial support from the German Research Foundation (HA4348/2-2 and HA4348/6-2 KFO311), the Federal Ministry of Education and Research (BMBF ViP+ program-03VP08053; BMBF 01KC2001B), and the European Pediatric Pulmonary Vascular Disease Network. Dr Calvier and Dr Herz are shareholders of Reelin Therapeutics and co-inventors of a patent related to anti-Reelin strategies (application number 15/763,047 and publication number 20180273637). Dr Hansmann has reported that he has no relationships relevant to the contents of this paper to disclose.
Figures




Similar articles
-
LRP1 Deficiency in Vascular SMC Leads to Pulmonary Arterial Hypertension That Is Reversed by PPARγ Activation.Circ Res. 2019 Jun 7;124(12):1778-1785. doi: 10.1161/CIRCRESAHA.119.315088. Epub 2019 Apr 26. Circ Res. 2019. PMID: 31023188 Free PMC article.
-
Postprandial lipoproteins and the molecular regulation of vascular homeostasis.Prog Lipid Res. 2013 Oct;52(4):446-64. doi: 10.1016/j.plipres.2013.06.001. Epub 2013 Jun 15. Prog Lipid Res. 2013. PMID: 23774609 Review.
-
Berberine attenuates hypoxia-induced pulmonary arterial hypertension via bone morphogenetic protein and transforming growth factor-β signaling.J Cell Physiol. 2019 Aug;234(10):17482-17493. doi: 10.1002/jcp.28370. Epub 2019 Feb 20. J Cell Physiol. 2019. PMID: 30786011
-
The LDL receptor-related protein (LRP) family: an old family of proteins with new physiological functions.Ann Med. 2007;39(3):219-28. doi: 10.1080/07853890701214881. Ann Med. 2007. PMID: 17457719 Review.
-
Functional role of lipoprotein receptors in Alzheimer's disease.Curr Alzheimer Res. 2008 Feb;5(1):15-25. doi: 10.2174/156720508783884675. Curr Alzheimer Res. 2008. PMID: 18288927 Review.
Cited by
-
The Roles of Fibrinolytic Factors in Bone Destruction Caused by Inflammation.Cells. 2024 Mar 15;13(6):516. doi: 10.3390/cells13060516. Cells. 2024. PMID: 38534360 Free PMC article. Review.
-
Genetic or therapeutic disruption of the Reelin/Apoer2 signaling pathway improves inflammatory arthritis outcomes.Proc Natl Acad Sci U S A. 2025 Mar 18;122(11):e2418642122. doi: 10.1073/pnas.2418642122. Epub 2025 Mar 12. Proc Natl Acad Sci U S A. 2025. PMID: 40073057
-
Oxidative Stress and Lipid Accumulation Augments Cell Death in LDLR-Deficient RPE Cells and Ldlr-/- Mice.Cells. 2022 Dec 22;12(1):43. doi: 10.3390/cells12010043. Cells. 2022. PMID: 36611838 Free PMC article.
-
Metabolic Regulation in Acute Respiratory Distress Syndrome: Implications for Inflammation and Oxidative Stress.Int J Chron Obstruct Pulmon Dis. 2025 Feb 18;20:373-388. doi: 10.2147/COPD.S491687. eCollection 2025. Int J Chron Obstruct Pulmon Dis. 2025. PMID: 39991071 Free PMC article. Review.
-
Whole Exome Sequencing Revealed Variants That Predict Pulmonary Artery Involvement in Patients with Takayasu Arteritis.J Inflamm Res. 2022 Aug 24;15:4817-4831. doi: 10.2147/JIR.S377402. eCollection 2022. J Inflamm Res. 2022. PMID: 36046661 Free PMC article.
References
-
- Beffert U., Stolt P.C., Herz J. Functions of lipoprotein receptors in neurons. J Lipid Res. 2004;45:403–409. - PubMed
-
- Hussain M.M., Strickland D.K., Bakillah A. The mammalian low-density lipoprotein receptor family. Annu Rev Nutr. 1999;19:141–172. - PubMed
-
- Willnow T.E., Christ A., Hammes A. Endocytic receptor-mediated control of morphogen signaling. Development. 2012;139:4311–4319. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

