A guide into the world of high-resolution 3D imaging: the case of soft X-ray tomography for the life sciences
- PMID: 35257156
- PMCID: PMC9162464
- DOI: 10.1042/BST20210886
A guide into the world of high-resolution 3D imaging: the case of soft X-ray tomography for the life sciences
Abstract
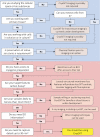
In the world of bioimaging, every choice made determines the quality and content of the data collected. The choice of imaging techniques for a study could showcase or dampen expected outcomes. Synchrotron radiation is indispensable for biomedical research, driven by the need to see into biological materials and capture intricate biochemical and biophysical details at controlled environments. The same need drives correlative approaches that enable the capture of heterologous but complementary information when studying any one single target subject. Recently, the applicability of one such synchrotron technique in bioimaging, soft X-ray tomography (SXT), facilitates exploratory and basic research and is actively progressing towards filling medical and industrial needs for the rapid screening of biomaterials, reagents and processes of immediate medical significance. Soft X-ray tomography at cryogenic temperatures (cryoSXT) fills the imaging resolution gap between fluorescence microscopy (in the hundreds of nanometers but relatively accessible) and electron microscopy (few nanometers but requires extensive effort and can be difficult to access). CryoSXT currently is accessible, fully documented, can deliver 3D imaging to 25 nm resolution in a high throughput fashion, does not require laborious sample preparation procedures and can be correlated with other imaging techniques. Here, we present the current state of SXT and outline its place within the bioimaging world alongside a guided matrix that aids decision making with regards to the applicability of any given imaging technique to a particular project. Case studies where cryoSXT has facilitated a better understanding of biological processes are highlighted and future directions are discussed.
© 2022 The Author(s).
Conflict of interest statement
The author declares that there are no competing interests associated with this manuscript.
Figures




References
-
- Eggeling, C. (2018) Advances in bioimaging—challenges and potentials. J. Phys. D: Appl. Phys. 51, 040201 10.1088/1361-6463/aaa259 - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

