Omadacycline efficacy in the hollow fibre system model of pulmonary Mycobacterium avium complex and potency at clinically attainable doses
- PMID: 35257162
- PMCID: PMC9155607
- DOI: 10.1093/jac/dkac068
Omadacycline efficacy in the hollow fibre system model of pulmonary Mycobacterium avium complex and potency at clinically attainable doses
Abstract
Objectives: The standard of care (SOC) for the treatment of pulmonary Mycobacterium avium complex (MAC) disease (clarithromycin, rifabutin, and ethambutol) achieves sustained sputum conversion rates of only 54%. Thus, new treatments should be prioritized.
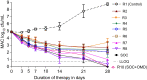
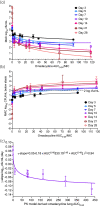
Methods: We identified the omadacycline MIC against one laboratory MAC strain and calculated drug half life in solution, which we compared with measured MAC doubling times. Next, we performed an omadacycline hollow fibre system model of intracellular MAC (HFS-MAC) exposure-effect study, as well as the three-drug SOC, using pharmacokinetics achieved in patient lung lesions. Data was analysed using bacterial kill slopes (γ-slopes) and inhibitory sigmoid Emax bacterial burden versus exposure analyses. Monte Carlo experiments (MCE) were used to identify the optimal omadacycline clinical dose.
Results: Omadacycline concentration declined in solution with a half-life of 27.7 h versus a MAC doubling time of 16.3 h, leading to artefactually high MICs. Exposures mediating 80% of maximal effect changed up to 8-fold depending on sampling day with bacterial burden versus exposure analyses, while γ-slope-based analyses gave a single robust estimate. The highest omadacycline monotherapy γ-slope was -0.114 (95% CI: -0.141 to -0.087) (r2 = 0.98) versus -0.114 (95% CI: -0.133 to -0.094) (r2 = 0.99) with the SOC. MCEs demonstrated that 450 mg of omadacycline given orally on the first 2 days followed by 300 mg daily would achieve the AUC0-24 target of 39.67 mg·h/L.
Conclusions: Omadacycline may be a potential treatment option for pulmonary MAC, possibly as a back-bone treatment for a new MAC regimen and warrants future study in treatment of this disease.
© The Author(s) 2022. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures



Similar articles
-
Sarecycline pharmacokinetics/pharmacodynamics in the hollow-fibre model of Mycobacterium avium complex: so near and yet so far.J Antimicrob Chemother. 2024 Jan 3;79(1):96-99. doi: 10.1093/jac/dkad352. J Antimicrob Chemother. 2024. PMID: 37946564 Free PMC article.
-
Ceftriaxone Efficacy for Mycobacterium avium Complex Lung Disease in the Hollow Fiber and Translation to Sustained Sputum Culture Conversion in Patients.J Infect Dis. 2024 Aug 16;230(2):e230-e240. doi: 10.1093/infdis/jiad545. J Infect Dis. 2024. PMID: 38036299 Free PMC article.
-
Ertapenem's therapeutic potential for Mycobacterium avium lung disease in the hollow fibre model.Int J Antimicrob Agents. 2024 Sep;64(3):107204. doi: 10.1016/j.ijantimicag.2024.107204. Epub 2024 May 15. Int J Antimicrob Agents. 2024. PMID: 38754528 Free PMC article.
-
Prevention strategies for Mycobacterium avium-intracellulare complex (MAC) infection. A review of recent studies in patients with AIDS.Drugs. 1997;54 Suppl 2:8-15; discussion 28-9. doi: 10.2165/00003495-199700542-00004. Drugs. 1997. PMID: 9358195 Review.
-
Risk-benefit assessment of therapies for Mycobacterium avium complex infections.Drug Saf. 1999 Aug;21(2):137-52. doi: 10.2165/00002018-199921020-00006. Drug Saf. 1999. PMID: 10456381 Review.
Cited by
-
Omadacycline drug susceptibility testing for non-tuberculous mycobacteria using oxyrase to overcome challenges with drug degradation.Tuberculosis (Edinb). 2024 Jul;147:102519. doi: 10.1016/j.tube.2024.102519. Epub 2024 May 13. Tuberculosis (Edinb). 2024. PMID: 38754247 Free PMC article.
-
Comparative efficacy of tetracyclines against isolates of Mycobacterium avium complex.IJTLD Open. 2025 Feb 1;2(2):113-115. doi: 10.5588/ijtldopen.24.0551. eCollection 2025 Feb. IJTLD Open. 2025. PMID: 39959406 Free PMC article. No abstract available.
-
Tigecycline pharmacodynamics in the hollow fiber system of Mycobacterium avium-complex lung disease, and the utility of MICs and time-kill studies in drug development.bioRxiv [Preprint]. 2025 Aug 1:2025.07.29.667481. doi: 10.1101/2025.07.29.667481. bioRxiv. 2025. PMID: 40766539 Free PMC article. Preprint.
-
Hollow-fibre system model of tuberculosis reproducibility and performance specifications for best practice in drug and combination therapy development.J Antimicrob Chemother. 2023 Apr 3;78(4):953-964. doi: 10.1093/jac/dkad029. J Antimicrob Chemother. 2023. PMID: 36794692 Free PMC article.
-
Drugs for treating infections caused by non-tubercular mycobacteria: a narrative review from the study group on mycobacteria of the Italian Society of Infectious Diseases and Tropical Medicine.Infection. 2024 Jun;52(3):737-765. doi: 10.1007/s15010-024-02183-3. Epub 2024 Feb 8. Infection. 2024. PMID: 38329686 Free PMC article. Review.
References
-
- Pasipanodya JG, Ogbonna D, Deshpande Det al. . Meta-analyses and the evidence base for microbial outcomes in the treatment of pulmonary Mycobacterium avium-intracellulare complex disease. J Antimicrob Chemother 2017; 72: i3–19. - PubMed
-
- Jeong B-H, Jeon K, Park HYet al. . Intermittent antibiotic therapy for nodular bronchiectatic Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2015; 191: 96–103. - PubMed
-
- Zweijpfenning S, Kops S, Magis-Escurra Cet al. . Treatment and outcome of non-tuberculous mycobacterial pulmonary disease in a predominantly fibro-cavitary disease cohort. Respir Med 2017; 131: 220–4. - PubMed

