Association between dosing and combination use of medications and outcomes in heart failure with reduced ejection fraction: data from the Swedish Heart Failure Registry
- PMID: 35257446
- PMCID: PMC9315143
- DOI: 10.1002/ejhf.2477
Association between dosing and combination use of medications and outcomes in heart failure with reduced ejection fraction: data from the Swedish Heart Failure Registry
Erratum in
-
Corrigendum to: 'An international Delphi consensus regarding best practice recommendations for hyperkalaemia across the cardiorenal spectrum' and articles listed below.Eur J Heart Fail. 2023 Mar;25(3):444. doi: 10.1002/ejhf.2790. Epub 2023 Feb 17. Eur J Heart Fail. 2023. PMID: 36799255 Free PMC article. No abstract available.
Abstract
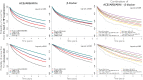
Aims: To assess the association between combination, dose and use of current guideline-recommended target doses (TD) of renin-angiotensin system inhibitors (RASi), angiotensin receptor-neprilysin inhibitors (ARNi) and β-blockers, and outcomes in a large and unselected contemporary cohort of patients with heart failure (HF) and reduced ejection fraction.
Methods and results: Overall, 17 809 outpatients registered in the Swedish Heart Failure Registry (SwedeHF) from May 2000 to December 2018, with ejection fraction <40% and duration of HF ≥90 days were selected. Primary outcome was a composite of time to cardiovascular death and first HF hospitalization. Compared with no use of RASi or ARNi, the adjusted hazard ratio (HR) (95% confidence interval [CI]) was 0.83 (0.76-0.91) with <50% of TD, 0.78 (0.71-0.86) with 50%-99%, and 0.73 (0.67-0.80) with ≥100% of TD. Compared with no use of β-blockers, the adjusted HR (95% CI) was 0.86 (0.76-0.91), 0.81 (0.74-0.89) and 0.74 (0.68-0.82) with <50%, 50%-99% and ≥100% of TD, respectively. Patients receiving both an angiotensin-converting enzyme inhibitor (ACEi)/angiotensin receptor blocker (ARB)/ARNi and a β-blocker at 50%-99% of TD had a lower adjusted risk of the primary outcome compared with patients only receiving one drug, i.e. ACEi/ARB/ARNi or β-blocker, even if this was at ≥100% of TD.
Conclusion: Heart failure with reduced ejection fraction patients using higher doses of RASi or ARNi and β-blockers had lower risk of cardiovascular death or HF hospitalization. Use of two drug classes at 50%-99% of TD dose was associated with lower risk than one drug class at 100% of TD.
Keywords: Heart failure; Implementation; Pharmacotherapy; Up-titration.
© 2022 The Authors. European Journal of Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Figures



Comment in
-
Uptitrating versus adding heart failure with reduced ejection fraction medications: bring more players to the game.Eur J Heart Fail. 2022 May;24(5):885-886. doi: 10.1002/ejhf.2507. Epub 2022 Apr 21. Eur J Heart Fail. 2022. PMID: 35417086 No abstract available.
-
Reply to: 'What is the optimal dose of neurohormonal modulators in patients with heart failure? The higher the better?'.Eur J Heart Fail. 2023 May;25(5):770-771. doi: 10.1002/ejhf.2837. Epub 2023 Apr 18. Eur J Heart Fail. 2023. PMID: 36987878 No abstract available.
References
-
- Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–55. - PubMed
-
- MERIT‐HF Study Group . Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT‐HF). Lancet. 1999;353:2001–7. - PubMed
-
- Poole‐Wilson PA. The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet. 1999;353:9–13. - PubMed
-
- Consensus Trial Study Group . Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

