Digitizing Chemical Synthesis in 3D Printed Reactionware
- PMID: 35257447
- PMCID: PMC9186708
- DOI: 10.1002/anie.202116108
Digitizing Chemical Synthesis in 3D Printed Reactionware
Abstract
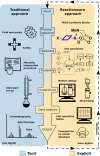
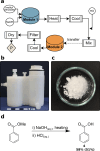
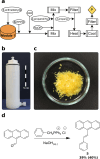
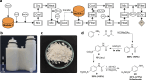
Chemistry digitization requires an unambiguous link between experiments and the code used to generate the experimental conditions and outcomes, yet this process is not standardized, limiting the portability of any chemical code. What is needed is a universal approach to aid this process using a well-defined standard that is composed of syntheses that are employed in modular hardware. Herein we present a new approach to the digitization of organic synthesis that combines process chemistry principles with 3D printed reactionware. This approach outlines the process for transforming unit operations into digitized hardware and well-defined instructions that ensure effective synthesis. To demonstrate this, we outline the process for digitizing 3 MIDA boronate building blocks, an ester hydrolysis, a Wittig olefination, a Suzuki-Miyaura coupling reaction, and synthesis of the drug sulfanilamide.
Keywords: 3D Printing; Chemical Education; C−C Coupling; Reactionware; Unit Operations.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
L.C. is the inventor on a patent entitled “Digital Reactionware” Publication number: 2020033518. The University of Illinois has filed patent applications related to MIDA boronates.
Figures