Photocurable antimicrobial silk-based hydrogels for corneal repair
- PMID: 35257514
- PMCID: PMC9313849
- DOI: 10.1002/jbm.a.37381
Photocurable antimicrobial silk-based hydrogels for corneal repair
Abstract
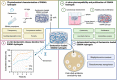
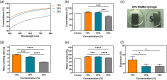
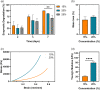
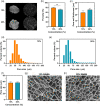
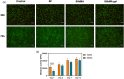
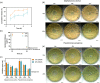
Corneal transplantation is the current gold standard treatment to restore visual acuity to patients with severe corneal diseases and injuries. Due to severe donor tissue shortage, efforts to develop a corneal equivalent have been made but the challenge remains unmet. Another issue of concern in ocular surgery is the difficult instillation and fast drainage of antibiotic ocular eye drops as bacterial infections can jeopardize implant success by delaying or impairing tissue healing. In this study, we developed antimicrobial silk-based hydrogels that have the potential to be photoactivated in situ, fully adapting to the corneal injury shape. Gentamicin-loaded methacrylated-silk (SilkMA) hydrogels were prepared within minutes using low UV intensity (3 mW/cm2 ). SilkMA gels provided a Young's modulus between 21 and 79 kPa together with a light transmittance spectrum and water content (83%-90%) similar to the human cornea. Polymer concentration (15%-25%) was found to offer a tool for tailoring the physical properties of the hydrogels. We confirmed that the methacrylation did not affect the material's in vitro degradation and biocompatibility by observing fibroblast adhesion and proliferation. Importantly, agar diffusion tests showed that the synthesized hydrogels were able to inhibit Staphylococcus aureus and Pseudomonas aeruginosa growth for 72 h. These characteristics along with their injectability and viscoelasticity demonstrate the potential of SilkMA hydrogels to be applied in several soft tissue engineering fields. As such, for the first time we demonstrate the potential of photocurable antimicrobial SilkMA hydrogels as a novel biomaterial to facilitate corneal regeneration.
Keywords: antimicrobial; cornea; regenerative medicine; silk fibroin.
© 2022 The Authors. Journal of Biomedical Materials Research Part A published by Wiley Periodicals LLC.
Figures

References
-
- Li L, Lu C, Wang L, et al. Gelatin‐based photocurable hydrogels for corneal wound repair. ACS Appl Mater Interfaces. 2018;10:13283‐13292. - PubMed
-
- Kilic Bektas C, Burcu A, Gedikoglu G, Telek HH, Ornek F, Hasirci V. Methacrylated gelatin hydrogels as corneal stroma substitutes: in vivo study. J Biomater Sci Polym Ed. 2019;30:1803‐1821. - PubMed
-
- Duarte Campos DF, Rohde M, Ross M, et al. Corneal bioprinting utilizing collagen‐based bioinks and primary human keratocytes. J Biomed Mater Res ‐ Part A. 2019;107:1945‐1953. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

