Mammalian monocarboxylate transporter 7 (MCT7/Slc16a6) is a novel facilitative taurine transporter
- PMID: 35257743
- PMCID: PMC8980330
- DOI: 10.1016/j.jbc.2022.101800
Mammalian monocarboxylate transporter 7 (MCT7/Slc16a6) is a novel facilitative taurine transporter
Abstract
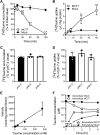
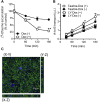
Monocarboxylate transporter 7 (MCT7) is an orphan transporter expressed in the liver, brain, and in several types of cancer cells. It has also been reported to be a survival factor in melanoma and breast cancers. However, this survival mechanism is not yet fully understood due to MCT7's unidentified substrate(s). Therefore, here we sought to identify MCT7 substrate(s) and characterize the transport mechanisms by analyzing amino acid transport in HEK293T cells and polarized Caco-2 cells. Analysis of amino acids revealed significant rapid reduction in taurine from cells transfected with enhanced green fluorescent protein-tagged MCT7. We found that taurine uptake and efflux by MCT7 was pH-independent and that the uptake was not saturated in the presence of taurine excess of 200 mM. Furthermore, we found that monocarboxylates and acidic amino acids inhibited MCT7-mediated taurine uptake. These results imply that MCT7 may be a low-affinity facilitative taurine transporter. We also found that MCT7 was localized at the basolateral membrane in polarized Caco-2 cells and that the induction of MCT7 expression in polarized Caco-2 cells enhanced taurine permeation. Finally, we demonstrated that interactions of MCT7 with ancillary proteins basigin/CD147 and embigin/GP70 enhanced MCT7-mediated taurine transport. In summary, these findings reveal that taurine is a novel substrate of MCT7 and that MCT7-mediated taurine transport might contribute to the efflux of taurine from cells.
Keywords: amino acid transport; efflux; facilitative transport; intestinal epithelium; membrane transport; monocarboxylate transporter 7; protein–protein interaction; taurine; transporter.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest The authors declare that they have no conflict of interest with the contents of this article.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

