Dopamine neurons exhibit emergent glutamatergic identity in Parkinson's disease
- PMID: 35258081
- PMCID: PMC9050538
- DOI: 10.1093/brain/awab373
Dopamine neurons exhibit emergent glutamatergic identity in Parkinson's disease
Abstract
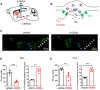
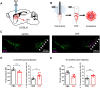
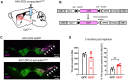
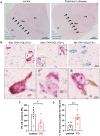
Loss of midbrain dopamine neurons causes the cardinal symptoms of Parkinson's disease. However, not all dopamine neurons are equally vulnerable and a better understanding of the cell-type specific properties relating to selective dopamine neuron degeneration is needed. Most midbrain dopamine neurons express the vesicular glutamate transporter VGLUT2 during development and a subset continue to express low levels of VGLUT2 in adulthood, enabling the co-release of glutamate. Moreover, VGLUT2 expression in dopamine neurons can be neuroprotective since its genetic disruption was shown to sensitize dopamine neurons to neurotoxins. Here, we show that in response to toxic insult, and in two distinct models of alpha-synuclein stress, VGLUT2 dopamine neurons were resilient to degeneration. Dopamine neurons expressing VGLUT2 were enriched whether or not insult induced dopamine neuron loss, suggesting that while VGLUT2 dopamine neurons are more resilient, VGLUT2 expression can also be transcriptionally upregulated by injury. Finally, we observed that VGLUT2 expression was enhanced in surviving dopamine neurons from post-mortem Parkinson's disease individuals. These data indicate that emergence of a glutamatergic identity in dopamine neurons may be part of a neuroprotective response in Parkinson's disease.
Keywords: Parkinson's disease; alpha-synuclein; dopamine; selective vulnerability; vesicular glutamate transporter 2.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Similar articles
-
Aging disrupts the coordination between mRNA and protein expression in mouse and human midbrain.Mol Psychiatry. 2025 Jul;30(7):3039-3054. doi: 10.1038/s41380-025-02909-1. Epub 2025 Jan 29. Mol Psychiatry. 2025. PMID: 39875589
-
Aging disrupts the coordination between mRNA and protein expression in mouse and human midbrain.bioRxiv [Preprint]. 2024 Jun 1:2024.06.01.596950. doi: 10.1101/2024.06.01.596950. bioRxiv. 2024. Update in: Mol Psychiatry. 2025 Jul;30(7):3039-3054. doi: 10.1038/s41380-025-02909-1. PMID: 38854057 Free PMC article. Updated. Preprint.
-
VGLUT2 Is a Determinant of Dopamine Neuron Resilience in a Rotenone Model of Dopamine Neurodegeneration.J Neurosci. 2021 Jun 2;41(22):4937-4947. doi: 10.1523/JNEUROSCI.2770-20.2021. Epub 2021 Apr 23. J Neurosci. 2021. PMID: 33893220 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Management of faecal incontinence and constipation in adults with central neurological diseases.Cochrane Database Syst Rev. 2013 Dec 18;(12):CD002115. doi: 10.1002/14651858.CD002115.pub4. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2014 Jan 13;(1):CD002115. doi: 10.1002/14651858.CD002115.pub5. PMID: 24347087 Updated.
Cited by
-
Natural Products and Their Neuroprotective Effects in Degenerative Brain Diseases: A Comprehensive Review.Int J Mol Sci. 2024 Oct 18;25(20):11223. doi: 10.3390/ijms252011223. Int J Mol Sci. 2024. PMID: 39457003 Free PMC article. Review.
-
Synaptic location is a determinant of the detrimental effects of α-synuclein pathology to glutamatergic transmission in the basolateral amygdala.Elife. 2022 Jul 1;11:e78055. doi: 10.7554/eLife.78055. Elife. 2022. PMID: 35775627 Free PMC article.
-
Roles of VGLUT2 and Dopamine/Glutamate Co-Transmission in Selective Vulnerability to Dopamine Neurodegeneration.ACS Chem Neurosci. 2022 Jan 19;13(2):187-193. doi: 10.1021/acschemneuro.1c00741. Epub 2022 Jan 7. ACS Chem Neurosci. 2022. PMID: 34994539 Free PMC article. Review.
-
Noninvasive Therapies: A Forthcoming Approach to Parkinson's Treatment.CNS Neurol Disord Drug Targets. 2025;24(3):165-180. doi: 10.2174/0118715273318429240812094557. CNS Neurol Disord Drug Targets. 2025. PMID: 39225218 Review.
-
Parkinson disease-associated toxic exposures selectively up-regulate vesicular glutamate transporter vGlut2 in a model of human cortical neurons.Mol Biol Cell. 2025 Feb 1;36(2):br4. doi: 10.1091/mbc.E24-08-0376. Epub 2025 Jan 2. Mol Biol Cell. 2025. PMID: 39745872 Free PMC article.

