Characterization of immune responses in fully vaccinated individuals after breakthrough infection with the SARS-CoV-2 delta variant
- PMID: 35258323
- PMCID: PMC8995036
- DOI: 10.1126/scitranslmed.abn6150
Characterization of immune responses in fully vaccinated individuals after breakthrough infection with the SARS-CoV-2 delta variant
Abstract
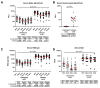
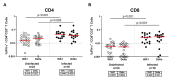
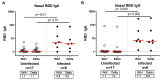
Breakthrough infections with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants have been reported frequently in vaccinated individuals with waning immunity. In particular, a cluster of over 1000 infections with the SARS-CoV-2 delta variant was identified in a predominantly fully vaccinated population in Provincetown, Massachusetts in July 2021. In this study, vaccinated individuals who tested positive for SARS-CoV-2 (n = 16) demonstrated substantially higher serum antibody responses than vaccinated individuals who tested negative for SARS-CoV-2 (n = 23), including 32-fold higher binding antibody titers and 31-fold higher neutralizing antibody titers against the SARS-CoV-2 delta variant. Vaccinated individuals who tested positive also showed higher mucosal antibody responses in nasal secretions and higher spike protein-specific CD8+ T cell responses in peripheral blood than did vaccinated individuals who tested negative. These data demonstrate that fully vaccinated individuals developed robust anamnestic antibody and T cell responses after infection with the SARS-CoV-2 delta variant. Moreover, these findings suggest that population immunity will likely increase over time by a combination of widespread vaccination and breakthrough infections.
Figures
Update of
-
Immune Responses in Fully Vaccinated Individuals Following Breakthrough Infection with the SARS-CoV-2 Delta Variant in Provincetown, Massachusetts.medRxiv [Preprint]. 2021 Oct 20:2021.10.18.21265113. doi: 10.1101/2021.10.18.21265113. medRxiv. 2021. Update in: Sci Transl Med. 2022 Apr 20;14(641):eabn6150. doi: 10.1126/scitranslmed.abn6150. PMID: 34704104 Free PMC article. Updated. Preprint.
References
-
- Brown C. M., Vostok J., Johnson H., Burns M., Gharpure R., Sami S., Sabo R. T., Hall N., Foreman A., Schubert P. L., Gallagher G. R., Fink T., Madoff L. C., Gabriel S. B., MacInnis B., Park D. J., Siddle K. J., Harik V., Arvidson D., Brock-Fisher T., Dunn M., Kearns A., Laney A. S., Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings - Barnstable County, Massachusetts, July 2021. MMWR Morb. Mortal. Wkly. Rep. 70, 1059–1062 (2021). 10.15585/mmwr.mm7031e2 - DOI - PMC - PubMed
-
- Baden L. R., El Sahly H. M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S. A., Rouphael N., Creech C. B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B. S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T.; COVE Study Group , Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 384, 403–416 (2021). 10.1056/NEJMoa2035389 - DOI - PMC - PubMed
-
- Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., Goepfert P. A., Truyers C., Fennema H., Spiessens B., Offergeld K., Scheper G., Taylor K. L., Robb M. L., Treanor J., Barouch D. H., Stoddard J., Ryser M. F., Marovich M. A., Neuzil K. M., Corey L., Cauwenberghs N., Tanner T., Hardt K., Ruiz-Guiñazú J., Le Gars M., Schuitemaker H., Van Hoof J., Struyf F., Douoguih M.; ENSEMBLE Study Group , Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 384, 2187–2201 (2021). 10.1056/NEJMoa2101544 - DOI - PMC - PubMed
-
- Polack F. P., Thomas S. J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J. L., Pérez Marc G., Moreira E. D., Zerbini C., Bailey R., Swanson K. A., Roychoudhury S., Koury K., Li P., Kalina W. V., Cooper D., Frenck R. W. Jr., Hammitt L. L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D. B., Mather S., Dormitzer P. R., Şahin U., Jansen K. U., Gruber W. C.; C4591001 Clinical Trial Group , Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 383, 2603–2615 (2020). 10.1056/NEJMoa2034577 - DOI - PMC - PubMed
-
- Fowlkes A., Gaglani M., Groover K., Thiese M. S., Tyner H., Ellingson K.; HEROES-RECOVER Cohorts , Effectiveness of COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Frontline Workers Before and During B.1.617.2 (Delta) Variant Predominance - Eight U.S. Locations, December 2020-August 2021. MMWR Morb. Mortal. Wkly. Rep. 70, 1167–1169 (2021). 10.15585/mmwr.mm7034e4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

