Comparison of 6-Month Outcomes of Survivors of COVID-19 versus Non-COVID-19 Critical Illness
- PMID: 35258437
- PMCID: PMC9872799
- DOI: 10.1164/rccm.202110-2335OC
Comparison of 6-Month Outcomes of Survivors of COVID-19 versus Non-COVID-19 Critical Illness
Erratum in
-
Erratum: Comparison of 6-Month Outcomes of Survivors of COVID-19 versus Non-COVID-19 Critical Illness.Am J Respir Crit Care Med. 2022 Sep 1;206(5):653. doi: 10.1164/rccm.v206erratum7. Am J Respir Crit Care Med. 2022. PMID: 36047765 Free PMC article. No abstract available.
Abstract
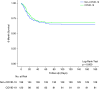
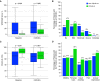
Rationale: The outcomes of survivors of critical illness due to coronavirus disease (COVID-19) compared with non-COVID-19 are yet to be established. Objectives: We aimed to investigate new disability at 6 months in mechanically ventilated patients admitted to Australian ICUs with COVID-19 compared with non-COVID-19. Methods: We included critically ill patients with COVID-19 and non-COVID-19 from two prospective observational studies. Patients were eligible if they were adult (age ⩾ 8 yr) and received ⩾24 hours of mechanical ventilation. In addition, patients with COVID-19 were eligible with a positive laboratory PCR test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Measurements and Main Results: Demographic, intervention, and hospital outcome data were obtained from electronic medical records. Survivors were contacted by telephone for functional outcomes with trained outcome assessors using the World Health Organization Disability Assessment Schedule 2.0. Between March 6, 2020, and April 21, 2021, 120 critically ill patients with COVID-19, and between August 2017 and January 2019, 199 critically ill patients without COVID-19, fulfilled the inclusion criteria. Patients with COVID-19 were older (median [interquartile range], 62 [55-71] vs. 58 [44-69] yr; P = 0.019) with a lower Acute Physiology and Chronic Health Evaluation II score (17 [13-20] vs. 19 [15-23]; P = 0.011). Although duration of ventilation was longer in patients with COVID-19 than in those without COVID-19 (12 [5-19] vs. 4.8 [2.3-8.8] d; P < 0.001), 180-day mortality was similar between the groups (39/120 [32.5%] vs. 70/199 [35.2%]; P = 0.715). The incidence of death or new disability at 180 days was similar (58/93 [62.4%] vs. 99/150 [66/0%]; P = 0.583). Conclusions: At 6 months, there was no difference in new disability for patients requiring mechanical ventilation for acute respiratory failure due to COVID-19 compared with non-COVID-19. Clinical trial registered with www.clinicaltrials.gov (NCT04401254).
Keywords: SARS-CoV-2; critical care; long COVID; long-term outcomes; recovery.
Figures

Comment in
-
Post-Intensive Care Syndrome in COVID-19 versus Non-COVID-19 Critical Illness Survivors: More Similar than Not?Am J Respir Crit Care Med. 2022 May 15;205(10):1133-1135. doi: 10.1164/rccm.202202-0396ED. Am J Respir Crit Care Med. 2022. PMID: 35380942 Free PMC article. No abstract available.
References
-
- Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Canadian Critical Care Trials Group Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med . 2011;364:1293–1304. - PubMed
-
- Geense WW, Zegers M, Peters MAA, Ewalds E, Simons KS, Vermeulen H, et al. New physical, mental, and cognitive problems 1 year after ICU admission: a prospective multicenter study. Am J Respir Crit Care Med . 2021;203:1512–1521. - PubMed
-
- Hodgson CL, Udy AA, Bailey M, Barrett J, Bellomo R, Bucknall T, et al. The impact of disability in survivors of critical illness. Intensive Care Med . 2017;43:992–1001. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

