Functional vulnerability of liver macrophages to capsules defines virulence of blood-borne bacteria
- PMID: 35258552
- PMCID: PMC8908791
- DOI: 10.1084/jem.20212032
Functional vulnerability of liver macrophages to capsules defines virulence of blood-borne bacteria
Abstract
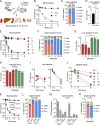
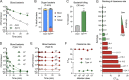
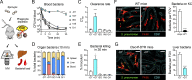
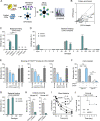
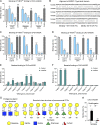
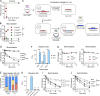
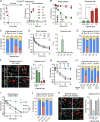
Many encapsulated bacteria use capsules to cause invasive diseases. However, it remains largely unknown how the capsules enhance bacterial virulence under in vivo infection conditions. Here we show that the capsules primarily target the liver to enhance bacterial survival at the onset of blood-borne infections. In a mouse sepsis model, the capsules enabled human pathogens Streptococcus pneumoniae and Escherichia coli to circumvent the recognition of liver-resident macrophage Kupffer cells (KCs) in a capsular serotype-dependent manner. In contrast to effective capture of acapsular bacteria by KCs, the encapsulated bacteria are partially (low-virulence types) or completely (high-virulence types) "untouchable" for KCs. We finally identified the asialoglycoprotein receptor (ASGR) as the first known capsule receptor on KCs to recognize the low-virulence serotype-7F and -14 pneumococcal capsules. Our data identify the molecular interplay between the capsules and KCs as a master controller of the fate and virulence of encapsulated bacteria, and suggest that the interplay is targetable for therapeutic control of septic infections.
© 2022 An et al.
Conflict of interest statement
Disclosures: M.R. Oggioni has a PhD student jointly with GSK Vaccines working on the pathogenesis of pneumonia (BBSRC grant BB/S507052/1). No other disclosures were reported.
Figures








References
-
- Aoyagi, Y., Adderson E.E., Min J.G., Matsushita M., Fujita T., Takahashi S., Okuwaki Y., and Bohnsack J.F.. 2005. Role of L-ficolin/mannose-binding lectin-associated serine protease complexes in the opsonophagocytosis of type III group B streptococci. J. Immunol. 174:418–425. 10.4049/jimmunol.174.1.418 - DOI - PubMed
-
- Aoyagi, Y., Adderson E.E., Rubens C.E., Bohnsack J.F., Min J.G., Matsushita M., Fujita T., Okuwaki Y., and Takahashi S.. 2008. L-Ficolin/Mannose-binding lectin-associated serine protease complexes bind to group B streptococci primarily through N-acetylneuraminic acid of capsular polysaccharide and activate the complement pathway. Infect. Immun. 76:179–188. 10.1128/IAI.00837-07 - DOI - PMC - PubMed
-
- Arshad, M., Goller C.C., Pilla D., Schoenen F.J., and Seed P.C.. 2016. Threading the needle: Small-molecule targeting of a xenobiotic receptor to ablate Escherichia coli polysaccharide capsule expression without altering antibiotic resistance. J. Infect. Dis. 213:1330–1339. 10.1093/infdis/jiv584 - DOI - PMC - PubMed
-
- Avery, O.T., Macleod C.M., McCarty M., and Peltier L. 2000. Studies on the chemical nature of the substance inducing transformation of pneumococcal types: Induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 79:137–158. 10.1097/00003086-200010001-00002 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

