Magnetic Resonance Imaging in Primary Progressive Multiple Sclerosis Patients : Review
- PMID: 35258820
- PMCID: PMC9424179
- DOI: 10.1007/s00062-022-01144-3
Magnetic Resonance Imaging in Primary Progressive Multiple Sclerosis Patients : Review
Abstract
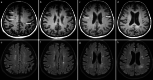
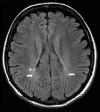
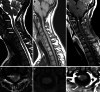
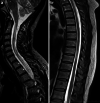
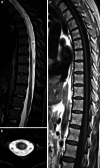
The recently developed effective treatment of primary progressive multiple sclerosis (PPMS) requires the accurate diagnosis of patients with this type of disease. Currently, the diagnosis of PPMS is based on the 2017 McDonald criteria, although the contribution of magnetic resonance imaging (MRI) to this process is fundamental. PPMS, one of the clinical types of MS, represents 10%-15% of all MS patients. Compared to relapsing-remitting MS (RRMS), PPMS differs in terms of pathology, clinical presentation and MRI features. Regarding conventional MRI, focal lesions on T2-weighted images and acute inflammatory lesions with contrast enhancement are less common in PPMS than in RRMS. On the other hand, MRI features of chronic inflammation, such as slowly evolving/expanding lesions (SELs) and leptomeningeal enhancement (LME), and brain and spinal cord atrophy are more common MRI characteristics in PPMS than RRMS. Nonconventional MRI also shows differences in subtle white and grey matter damage between PPMS and other clinical types of disease. In this review, we present separate diagnostic criteria, conventional and nonconventional MRI specificity for PPMS, which may support and simplify the diagnosis of this type of MS in daily clinical practice.
Keywords: Diagnosis of multiple sclerosis; Differential diagnosis.
© 2022. The Author(s).
Conflict of interest statement
The author declares that there is no conflict of interest.
Figures

References
-
- Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, de Seze J, Giovannoni G, Hartung HP, Hemmer B, Lublin F, Rammohan KW, Selmaj K, Traboulsee A, Sauter A, Masterman D, Fontoura P, Belachew S, Garren H, Mairon N, Chin P, Wolinsky JS, ORATORIO Clinical Investigators Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;19:209–220. doi: 10.1056/NEJMoa1606468. - DOI - PubMed
-
- Wolinsky JS, Arnold DL, Brochet B, Hartung HP, Montalban X, Naismith RT, Manfrini M, Overell J, Koendgen H, Sauter A, Bennett I, Hubeaux S, Kappos L, Hauser SL. Long-term follow-up from the ORATORIO trial of ocrelizumab for primary progressive multiple sclerosis: a post-hoc analysis from the ongoing open-label extension of the randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2020;19:998–1009. doi: 10.1016/S1474-4422(20)30342-2. - DOI - PubMed
-
- Hawker K, O’Connor P, Freedman MS, Calabresi PA, Antel J, Simon J, Hauser S, Waubant E, Vollmer T, Panitch H, Zhang J, Chin P, Smith CH, OLYMPUS trial group Rituximab in patients with primary progressive multiple sclerosis: results of arandomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66:460–471. doi: 10.1002/ana.21867. - DOI - PubMed
-
- Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, Sorensen PS, Tintoré M, Traboulsee AL, Trojano M, Uitdehaag BMJ, Vukusic S, Waubant E, Weinshenker BG, Reingold SC, Cohen JA. Diagnosis of multiple sclerosis: 2017revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. doi: 10.1016/S1474-4422(17)30470-2. - DOI - PubMed
-
- Filippi M, Rocca MA, Ciccarelli O, De Stefano N, Evangelou N, Kappos L, Rovira A, Sastre-Garriga J, Tintorè M, Frederiksen JL, Gasperini C, Palace J, Reich DS, Banwell B, Montalban X, Barkhof F, MAGNIMS Study Group MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 2016;15:292–303. doi: 10.1016/S1474-4422(15)00393-2. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

