Mechanism of Radical S-Adenosyl- l-methionine Adenosylation: Radical Intermediates and the Catalytic Competence of the 5'-Deoxyadenosyl Radical
- PMID: 35258967
- PMCID: PMC9524473
- DOI: 10.1021/jacs.1c13706
Mechanism of Radical S-Adenosyl- l-methionine Adenosylation: Radical Intermediates and the Catalytic Competence of the 5'-Deoxyadenosyl Radical
Abstract
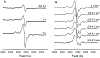
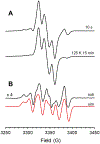
Radical S-adenosyl-l-methionine (SAM) enzymes employ a [4Fe-4S] cluster and SAM to initiate diverse radical reactions via either H-atom abstraction or substrate adenosylation. Here we use freeze-quench techniques together with electron paramagnetic resonance (EPR) spectroscopy to provide snapshots of the reaction pathway in an adenosylation reaction catalyzed by the radical SAM enzyme pyruvate formate-lyase activating enzyme on a peptide substrate containing a dehydroalanine residue in place of the target glycine. The reaction proceeds via the initial formation of the organometallic intermediate Ω, as evidenced by the characteristic EPR signal with g∥ = 2.035 and g⊥ = 2.004 observed when the reaction is freeze-quenched at 500 ms. Thermal annealing of frozen Ω converts it into a second paramagnetic species centered at giso = 2.004; this second species was generated directly using freeze-quench at intermediate times (∼8 s) and unequivocally identified via isotopic labeling and EPR spectroscopy as the tertiary peptide radical resulting from adenosylation of the peptide substrate. An additional paramagnetic species observed in samples quenched at intermediate times was revealed through thermal annealing while frozen and spectral subtraction as the SAM-derived 5'-deoxyadenosyl radical (5'-dAdo•). The time course of the 5'-dAdo• and tertiary peptide radical EPR signals reveals that the former generates the latter. These results thus support a mechanism in which Ω liberates 5'-dAdo• by Fe-C5' bond homolysis, and the 5'-dAdo• attacks the dehydroalanine residue of the peptide substrate to form the adenosylated peptide radical species. The results thus provide a picture of a catalytically competent 5'-dAdo• intermediate trapped just prior to reaction with the substrate.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
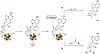
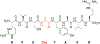

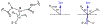
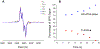
References
-
- Frey PA; Hegeman AD; Ruzicka FJ The radical SAM superfamily. Crit. Rev. Biochem. Mol. Biol 2008, 43, 63–88. - PubMed
-
- Landgraf BJ; McCarthy EL; Booker SJ Radical S-adenosylmethionine enzymes in human health and disease. Annu. Rev. Biochem 2016, 85, 485–514. - PubMed
-
- Bridwell-Rabb J; Grell TAJ; Drennan CL A rich man, poor man story of S-adenosylmethionine and cobalamin revisited. Annu. Rev. Biochem 2018, 87, 555–584. - PubMed
-
- Nicolet Y Structure-function relationships of radical of radical SAM enzymes. Nat. Catal 2020, 3, 337–350.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

