Lung Cancer Diagnosed Through Screening, Lung Nodule, and Neither Program: A Prospective Observational Study of the Detecting Early Lung Cancer (DELUGE) in the Mississippi Delta Cohort
- PMID: 35258994
- PMCID: PMC9242408
- DOI: 10.1200/JCO.21.02496
Lung Cancer Diagnosed Through Screening, Lung Nodule, and Neither Program: A Prospective Observational Study of the Detecting Early Lung Cancer (DELUGE) in the Mississippi Delta Cohort
Abstract
Purpose: Lung cancer screening saves lives, but implementation is challenging. We evaluated two approaches to early lung cancer detection-low-dose computed tomography screening (LDCT) and program-based management of incidentally detected lung nodules.
Methods: A prospective observational study enrolled patients in the early detection programs. For context, we compared them with patients managed in a Multidisciplinary Care Program. We compared clinical stage distribution, surgical resection rates, 3- and 5-year survival rates, and eligibility for LDCT screening of patients diagnosed with lung cancer.
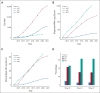
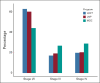
Results: From 2015 to May 2021, 22,886 patients were enrolled: 5,659 in LDCT, 15,461 in Lung Nodule, and 1,766 in Multidisciplinary Care. Of 150, 698, and 1,010 patients diagnosed with lung cancer in the respective programs, 61%, 60%, and 44% were diagnosed at clinical stage I or II, whereas 19%, 20%, and 29% were stage IV (P = .0005); 47%, 42%, and 32% had curative-intent surgery (P < .0001); aggregate 3-year overall survival rates were 80% (95% CI, 73 to 88) versus 64% (60 to 68) versus 49% (46 to 53); 5-year overall survival rates were 76% (67 to 87) versus 60% (56 to 65) versus 44% (40 to 48), respectively. Only 46% of 1,858 patients with lung cancer would have been deemed eligible for LDCT by US Preventive Services Task Force (USPSTF) 2013 criteria, and 54% by 2021 criteria. Even if all eligible patients by USPSTF 2021 criteria had been enrolled into LDCT, the Nodule Program would have detected 20% of the stage I-II lung cancer in the entire cohort.
Conclusion: LDCT and Lung Nodule Programs are complementary, expanding access to early lung cancer detection and curative treatment to different-risk populations. Implementing Lung Nodule Programs may alleviate emerging disparities in access to early lung cancer detection.
Conflict of interest statement
Figures


Comment in
-
Complementary Approaches to Lung Cancer Detection in High-Risk Populations.J Clin Oncol. 2022 Jul 1;40(19):2074-2077. doi: 10.1200/JCO.22.00494. Epub 2022 May 23. J Clin Oncol. 2022. PMID: 35605172 No abstract available.
References
-
- Siegel RL, Miller KD, Fuchs HE, et al. : Cancer statistics, 2021. CA Cancer J Clin 71:7-33, 2021. [Erratum: CA Cancer J Clin 71:359, 2021] - PubMed
-
- de Koning HJ, van der Aalst CM, de Jong PA, et al. : Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 382:503-513, 2020 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

