T-cadherin Expressing Cells in the Stromal Vascular Fraction of Human Adipose Tissue: Role in Osteogenesis and Angiogenesis
- PMID: 35259280
- PMCID: PMC8929526
- DOI: 10.1093/stcltm/szab021
T-cadherin Expressing Cells in the Stromal Vascular Fraction of Human Adipose Tissue: Role in Osteogenesis and Angiogenesis
Abstract
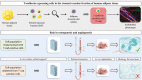
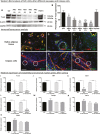
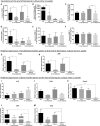
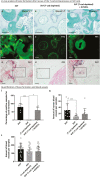
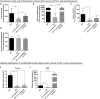
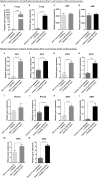
Cells of the stromal vascular fraction (SVF) of human adipose tissue have the capacity to generate osteogenic grafts with intrinsic vasculogenic properties. However, cultured adipose-derived stromal cells (ASCs), even after minimal monolayer expansion, lose osteogenic capacity in vivo. Communication between endothelial and stromal/mesenchymal cell lineages has been suggested to improve bone formation and vascularization by engineered tissues. Here, we investigated the specific role of a subpopulation of SVF cells positive for T-cadherin (T-cad), a putative endothelial marker. We found that maintenance during monolayer expansion of a T-cad-positive cell population, composed of endothelial lineage cells (ECs), is mandatory to preserve the osteogenic capacity of SVF cells in vivo and strongly supports their vasculogenic properties. Depletion of T-cad-positive cells from the SVF totally impaired bone formation in vivo and strongly reduced vascularization by SVF cells in association with decreased VEGF and Adiponectin expression. The osteogenic potential of T-cad-depleted SVF cells was fully rescued by co-culture with ECs from a human umbilical vein (HUVECs), constitutively expressing T-cad. Ectopic expression of T-cad in ASCs stimulated mineralization in vitro but failed to rescue osteogenic potential in vivo, indicating that the endothelial nature of the T-cad-positive cells is the key factor for induction of osteogenesis in engineered grafts based on SVF cells. This study demonstrates that crosstalk between stromal and T-cad expressing endothelial cells within adipose tissue critically regulates osteogenesis, with VEGF and adiponectin as associated molecular mediators.
Keywords: 3D microenvironment; adipose stem cells; angiogenesis; blood vessels; bone; osteogenesis.
© The Author(s) 2022. Published by Oxford University Press.
Figures


References
-
- Barabaschi GD, Manoharan V, Li Q, Bertassoni LE. Engineering pre-vascularized scaffolds for bone regeneration. Adv Exp Med Biol. 2015;881:79-94. - PubMed
-
- Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends Biotechnol. 2008;26(8):434-441. - PubMed
-
- Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011;63(4-5):300-311. - PubMed
-
- Miranville A, Heeschen C, Sengenès C, Curat CA, Busse R, Bouloumié A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110(3):349-355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

