Hepatic recruitment of eosinophils and their protective function during acute liver injury
- PMID: 35259470
- PMCID: PMC9308653
- DOI: 10.1016/j.jhep.2022.02.024
Hepatic recruitment of eosinophils and their protective function during acute liver injury
Abstract
Background & aims: Beyond the classical description of eosinophil functions in parasite infections and allergic diseases, emerging evidence supports a critical role of eosinophils in resolving inflammation and promoting tissue remodeling. However, the role of eosinophils in liver injury and the underlying mechanism of their recruitment into the liver remain unclear.
Methods: Hepatic eosinophils were detected and quantified using flow cytometry and immunohistochemical staining. Eosinophil-deficient (ΔdblGata1) mice were used to investigate the role of eosinophils in 3 models of acute liver injury. In vivo experiments using Il33-/- mice and macrophage-depleted mice, as well as in vitro cultures of eosinophils and macrophages, were performed to interrogate the mechanism of eotaxin-2 (CCL24) production.
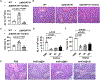
Results: Hepatic accumulation of eosinophils was observed in patients with acetaminophen (APAP)-induced liver failure, whereas few eosinophils were detectable in healthy liver tissues. In mice treated with APAP, carbon tetrachloride or concanavalin A, eosinophils were recruited into the liver and played a profound protective role. Mice deficient of macrophages or IL-33 exhibited impaired hepatic eosinophil recruitment during acute liver injury. CCL24, but not CCL11, was increased after treatment of each hepatotoxin in an IL-33 and macrophage-dependent manner. In vitro experiments demonstrated that IL-33, by stimulating IL-4 release from eosinophils, promoted the production of CCL24 by macrophages.
Conclusions: This is the first study to demonstrate that hepatic recruitment of and protection by eosinophils occur commonly in various models of acute liver injury. Our findings support further exploration of eosinophils as a therapeutic target to treat APAP-induced acute liver injury.
Lay summary: The current study unveils that eosinophils are recruited into the liver and play a protective function during acute liver injury caused by acetaminophen overdose. The data demonstrate that IL-33-activated eosinophils trigger macrophages to release high amounts of CCL24, which promotes hepatic eosinophil recruitment. Our findings suggest that eosinophils could be an effective cell-based therapy for the treatment of acetaminophen-induced acute liver injury.
Keywords: CCL24; Eosinophils; IL-33; acute liver injury; macrophages.
Copyright © 2022 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no competing interests. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


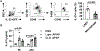
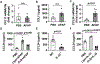
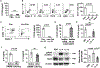
References
-
- Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. The Journal of allergy and clinical immunology. 2004;113(1):30–7. - PubMed
-
- Gleich GJ. Mechanisms of eosinophil-associated inflammation. The Journal of allergy and clinical immunology. 2000;105(4):651–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

