Cell cycle checkpoints and beyond: Exploiting the ATR/CHK1/WEE1 pathway for the treatment of PARP inhibitor-resistant cancer
- PMID: 35259479
- PMCID: PMC9026671
- DOI: 10.1016/j.phrs.2022.106162
Cell cycle checkpoints and beyond: Exploiting the ATR/CHK1/WEE1 pathway for the treatment of PARP inhibitor-resistant cancer
Abstract
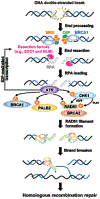
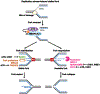
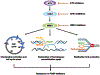
Poly (ADP-ribose) polymerase (PARP) inhibitors (PARPis) have become a mainstay of therapy in ovarian cancer and other malignancies, including BRCA-mutant breast, prostate, and pancreatic cancers. However, a growing number of patients develop resistance to PARPis, highlighting the need to further understand the mechanisms of PARPi resistance and develop effective treatment strategies. Targeting cell cycle checkpoint protein kinases, e.g., ATR, CHK1, and WEE1, which are upregulated in response to replication stress, represents one such therapeutic approach for PARPi-resistant cancers. Mechanistically, activated cell cycle checkpoints promote cell cycle arrest, replication fork stabilization, and DNA repair, demonstrating the interplay of DNA repair proteins with replication stress in the development of PARPi resistance. Inhibitors of these cell cycle checkpoints are under investigation in PARPi-resistant ovarian and other cancers. In this review, we discuss the cell cycle checkpoints and their roles beyond mere cell cycle regulation as part of the arsenal to overcome PARPi-resistant cancers. We also address the current status and recent advancements as well as limitations of cell cycle checkpoint inhibitors in clinical trials.
Keywords: ATR/CHK1/WEE1 pathway; Cell cycle checkpoint; DNA damage response; Ovarian cancer; PARP inhibitor resistance; Replication stress.
Published by Elsevier Ltd.
Conflict of interest statement
Conflicts of Interest:
The authors declare no potential conflicts of interest.
Figures

References
-
- Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke GS, Gourley C, Banerjee S, Oza A, Gonzalez-Martin A, Aghajanian C, Bradley W, Mathews C, Liu J, Lowe ES, Bloomfield R, DiSilvestro P, Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer, N. Engl. J. Med 379 (2018) 2495–2505. - PubMed
-
- Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, Fehrenbacher L, Yerushalmi R, Mina LA, Martin M, Roche H, Im YH, Quek RGW, Markova D, Tudor IC, Hannah AL, Eiermann W, Blum JL, Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation, N. Engl. J. Med 379 (2018) 753–763. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

