Early administration of tocilizumab in hospitalized COVID-19 patients with elevated inflammatory markers; COVIDSTORM-a prospective, randomized, single-centre, open-label study
- PMID: 35259529
- PMCID: PMC8897958
- DOI: 10.1016/j.cmi.2022.02.027
Early administration of tocilizumab in hospitalized COVID-19 patients with elevated inflammatory markers; COVIDSTORM-a prospective, randomized, single-centre, open-label study
Abstract
Objectives: Severe COVID-19 is associated with an imbalanced immune response. We hypothesized that patients with enhanced inflammation, as demonstrated by increased levels of certain inflammatory biomarkers, would benefit from interleukin-6 blockage.
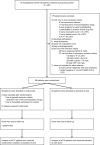
Methods: Patients hospitalized with COVID-19, hypoxemia, and at least two of four markedly elevated markers of inflammation (interleukin-6, C-reactive protein, ferritin, and/or D-dimer) were randomized for tocilizumab (TCZ) plus standard of care (SoC) or SoC alone. The primary endpoint was clinical status at day 28 assessed using a seven-category ordinal scale, and the secondary endpoints included intensive care unit admission, respiratory support, and duration of hospital admission.
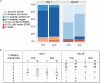
Results: Clinical status at day 28 was significantly better in patients who received TCZ in addition to SoC compared with those who received SoC alone (p = 0.037). By then, 93% of patients who received TCZ (n = 53 of 57) and 86% of control patients (n = 25 of 29) had been discharged from the hospital. In addition, 47% of TCZ patients (n = 27 of 57) and 24% of control patients (n = 7 of 29) had resumed normal daily activities. The median length of hospitalization was 9 days (interquartile range, 7-12) in the TCZ group and 12 days (interquartile range, 9-15) in the control group (p = 0.014).
Discussion: In patients hospitalized with COVID-19, hypoxemia, and elevated inflammation markers, administration of TCZ in addition to SoC was associated with significantly better clinical recovery by day 28 and a shorter hospitalization compared with SoC alone.
Keywords: COVID-19; Hospitalization; Interleukin-6; SARS-CoV-2; Tocilizumab.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Re: Early administration of tocilizumab in hospitalized COVID-19 patients with elevated inflammatory markers; COVIDSTORM.Clin Microbiol Infect. 2022 Nov;28(11):1519. doi: 10.1016/j.cmi.2022.06.013. Epub 2022 Jun 20. Clin Microbiol Infect. 2022. PMID: 35738319 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

