The functional properties of synapses made by regenerated axons across spinal cord lesion sites in lamprey
- PMID: 35259849
- PMCID: PMC9083143
- DOI: 10.4103/1673-5374.335828
The functional properties of synapses made by regenerated axons across spinal cord lesion sites in lamprey
Abstract
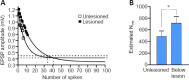
While the anatomical properties of regenerated axons across spinal cord lesion sites have been studied extensively, little is known of how the functional properties of regenerated synapses compared to those in unlesioned animals. This study aims to compare the properties of synapses made by regenerated axons with unlesioned axons using the lamprey, a model system for spinal injury research, in which functional locomotor recovery after spinal cord lesions is associated with axonal regeneration across the lesion site. Regenerated synapses below the lesion site did not differ from synapses from unlesioned axons with respect to the amplitude and duration of single excitatory postsynaptic potentials. They also showed the same activity-dependent depression over spike trains. However, regenerated synapses did differ from unlesioned synapses as the estimated number of synaptic vesicles was greater and there was evidence for increased postsynaptic quantal amplitude. For axons above the lesion site, the amplitude and duration of single synaptic inputs also did not differ significantly from unlesioned animals. However, in this case, there was evidence of a reduction in release probability and inputs facilitated rather than depressed over spike trains. Synaptic inputs from single regenerated axons below the lesion site thus do not increase in amplitude to compensate for the reduced number of descending axons after functional recovery. However, the postsynaptic input was maintained at the unlesioned level using different synaptic properties. Conversely, the facilitation from the same initial amplitude above the lesion site made the synaptic input over spike trains functionally stronger. This may help to increase propriospinal activity across the lesion site to compensate for the lesion-induced reduction in supraspinal inputs. The animal experiments were approved by the Animal Ethics Committee of Cambridge University.
Keywords: electrophysiology; lamprey; plasticity; regeneration; reticulospinal axon; spinal cord; spinal injury; synapse.
Conflict of interest statement
None
Figures





References
-
- Becker M, Parker D. Time course of functional changes in locomotor and sensory systems after spinal cord lesions in lamprey. J Neurophysiol. 2019;121:2323–2335. - PubMed
-
- Berry M, Pentreath V. Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res. 1976;105:1–20. - PubMed
LinkOut - more resources
Full Text Sources

