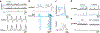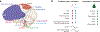Breathing Rhythm and Pattern and Their Influence on Emotion
- PMID: 35259917
- PMCID: PMC9840384
- DOI: 10.1146/annurev-neuro-090121-014424
Breathing Rhythm and Pattern and Their Influence on Emotion
Abstract
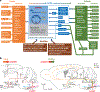
Breathing is a vital rhythmic motor behavior with a surprisingly broad influence on the brain and body. The apparent simplicity of breathing belies a complex neural control system, the breathing central pattern generator (bCPG), that exhibits diverse operational modes to regulate gas exchange and coordinate breathing with an array of behaviors. In this review, we focus on selected advances in our understanding of the bCPG. At the core of the bCPG is the preBötzinger complex (preBötC), which drives inspiratory rhythm via an unexpectedly sophisticated emergent mechanism. Synchronization dynamics underlying preBötC rhythmogenesis imbue the system with robustness and lability. These dynamics are modulated by inputs from throughout the brain and generate rhythmic, patterned activity that is widely distributed. The connectivity and an emerging literature support a link between breathing, emotion, and cognition that is becoming experimentally tractable. These advances bring great potential for elucidating function and dysfunction in breathing and other mammalian neural circuits.
Keywords: central pattern generators; emotion; motor systems; network dynamics; preBötzinger complex; synchrony.
Figures