Combination cancer immunotherapy targeting TNFR2 and PD-1/PD-L1 signaling reduces immunosuppressive effects in the microenvironment of pancreatic tumors
- PMID: 35260434
- PMCID: PMC8906048
- DOI: 10.1136/jitc-2021-003982
Combination cancer immunotherapy targeting TNFR2 and PD-1/PD-L1 signaling reduces immunosuppressive effects in the microenvironment of pancreatic tumors
Erratum in
-
Correction: Combination cancer immunotherapy targeting TNFR2 and PD-1/PD-L1 signaling reduces immunosuppressive effects in the microenvironment of pancreatic tumors.J Immunother Cancer. 2025 Apr 9;13(4):e003982corr1. doi: 10.1136/jitc-2021-003982corr1. J Immunother Cancer. 2025. PMID: 40210241 Free PMC article. No abstract available.
Abstract
Backgrounds: In advanced pancreatic ductal adenocarcinoma (PDAC), immune therapy, including immune checkpoint inhibitors, has limited efficacy, encouraging the study of combination therapy.
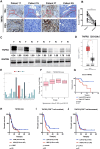
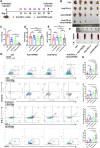
Methods: Tumor necrosis factor receptor 2 (TNFR2) was analyzed via immunohistochemistry, immunofluorescence, western blotting, and ELISAs. The in vitro mechanism that TNFR2 regulates programmed cell death 1 ligand 1 (PD-L1) was investigated using immunofluorescence, immunohistochemistry, flow cytometry, western blotting, and chromatin immunoprecipitation (ChIP). In vivo efficacy and mechanistic studies, using C57BL/6 mice and nude mice with KPC cell-derived subcutaneous and orthotopic tumors, employed antibodies against TNFR2 and PD-L1. Survival curves were constructed for the orthotopic model and a genetically engineered PDAC model (LSL-KrasG12D/+; LSL-Trp53R172H/+; Pdx1-Cre). Mass cytometry, immunohistochemistry, and flow cytometry analyzed local and systemic alterations in the immunophenotype.
Results: TNFR2 showed high expression and is a prognostic factor in CD8+ T cell-enriched pancreatic cancer. TNFR2 promotes tumorigenesis and progression of pancreatic cancer via dual effect: suppressing cancer immunogenicity and partially accelerating tumor growth. TNFR2 positivity correlated with PD-L1, and in vitro and in vivo, it could regulate the expression of PDL1 at the transcription level via the p65 NF-κB pathway. Combining anti-TNFR2 and PD-L1 antibodies eradicated tumors, prolonged overall survival in pancreatic cancer, and induced strong antitumor immune memory and secondary prevention by reducing the infiltration of Tregs and tumor-associated macrophages and inducing CD8+ T cell activation in the PDAC microenvironment. Finally, the antitumor immune response derived from combination therapy is mainly dependent on CD8+ T cells, partially dependent on CD4+ T cells, and independent of natural killer cells.
Conclusions: Anti-TNFR2 and anti-PD-L1 combination therapy eradicated tumors by inhibiting their growth, relieving tumor immunosuppression, and generating robust memory recall.
Keywords: B7-H1 antigen; biomarkers; gastrointestinal neoplasms; immunotherapy; tumor.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
