Adipocyte-derived PGE2 is required for intermittent fasting-induced Treg proliferation and improvement of insulin sensitivity
- PMID: 35260536
- PMCID: PMC8983131
- DOI: 10.1172/jci.insight.153755
Adipocyte-derived PGE2 is required for intermittent fasting-induced Treg proliferation and improvement of insulin sensitivity
Abstract
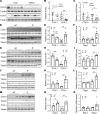
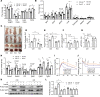
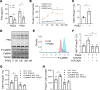
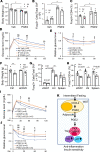
The intermittent fasting (IF) diet has profound benefits for diabetes prevention. However, the precise mechanisms underlying IF's beneficial effects remain poorly defined. Here, we show that the expression levels of cyclooxygenase-2 (COX-2), an enzyme that produces prostaglandins, are suppressed in white adipose tissue (WAT) of obese humans. In addition, the expression of COX-2 in WAT is markedly upregulated by IF in obese mice. Adipocyte-specific depletion of COX-2 led to reduced fractions of CD4+Foxp3+ Tregs and a substantial decrease in the frequency of CD206+ macrophages, an increase in the abundance of γδT cells in WAT under normal chow diet conditions, and attenuation of IF-induced antiinflammatory and insulin-sensitizing effects, despite a similar antiobesity effect in obese mice. Mechanistically, adipocyte-derived prostaglandin E2 (PGE2) promoted Treg proliferation through the CaMKII pathway in vitro and rescued Treg populations in adipose tissue in COX-2-deficient mice. Ultimately, inactivation of Tregs by neutralizing anti-CD25 diminished IF-elicited antiinflammatory and insulin-sensitizing effects, and PGE2 restored the beneficial effects of IF in COX-2-KO mice. Collectively, our study reveals that adipocyte COX-2 is a key regulator of Treg proliferation and that adipocyte-derived PGE2 is essential for IF-elicited type 2 immune response and metabolic benefits.
Keywords: Adipose tissue; Innate immunity; Metabolism.
Figures



Similar articles
-
Importance of adipocyte cyclooxygenase-2 and prostaglandin E2-prostaglandin E receptor 3 signaling in the development of obesity-induced adipose tissue inflammation and insulin resistance.FASEB J. 2016 Jun;30(6):2282-97. doi: 10.1096/fj.201500127. Epub 2016 Mar 1. FASEB J. 2016. PMID: 26932930
-
Prostaglandin E2 Exerts Multiple Regulatory Actions on Human Obese Adipose Tissue Remodeling, Inflammation, Adaptive Thermogenesis and Lipolysis.PLoS One. 2016 Apr 28;11(4):e0153751. doi: 10.1371/journal.pone.0153751. eCollection 2016. PLoS One. 2016. PMID: 27124181 Free PMC article.
-
The Tpl2 Kinase Regulates the COX-2/Prostaglandin E2 Axis in Adipocytes in Inflammatory Conditions.Mol Endocrinol. 2015 Jul;29(7):1025-36. doi: 10.1210/me.2015-1027. Epub 2015 May 28. Mol Endocrinol. 2015. PMID: 26020725 Free PMC article.
-
The Dualistic Effect of COX-2-Mediated Signaling in Obesity and Insulin Resistance.Int J Mol Sci. 2019 Jun 26;20(13):3115. doi: 10.3390/ijms20133115. Int J Mol Sci. 2019. PMID: 31247902 Free PMC article. Review.
-
Omega-3 fatty acids and adipose tissue biology.Mol Aspects Med. 2018 Dec;64:147-160. doi: 10.1016/j.mam.2018.01.004. Epub 2018 Jan 17. Mol Aspects Med. 2018. PMID: 29329795 Review.
Cited by
-
Acoustic modulation of mechanosensitive genes and adipocyte differentiation.Commun Biol. 2025 Apr 16;8(1):595. doi: 10.1038/s42003-025-07969-1. Commun Biol. 2025. PMID: 40240542 Free PMC article.
-
A Bibliometric and Visualization Analysis of Intermittent Fasting.Front Public Health. 2022 Jul 6;10:946795. doi: 10.3389/fpubh.2022.946795. eCollection 2022. Front Public Health. 2022. PMID: 35875030 Free PMC article.
-
Targeting metabolic pathways: a novel therapeutic direction for type 2 diabetes.Front Cell Infect Microbiol. 2023 Aug 2;13:1218326. doi: 10.3389/fcimb.2023.1218326. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37600949 Free PMC article.
-
Altered immunometabolic response to fasting in humans living with obesity.iScience. 2025 Jun 11;28(7):112872. doi: 10.1016/j.isci.2025.112872. eCollection 2025 Jul 18. iScience. 2025. PMID: 40662191 Free PMC article.
-
Epigenetic regulation of human FOXP3+ Tregs: from homeostasis maintenance to pathogen defense.Front Immunol. 2024 Jul 31;15:1444533. doi: 10.3389/fimmu.2024.1444533. eCollection 2024. Front Immunol. 2024. PMID: 39144146 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

