Prognostic Significance of Clinicopathological Factors Influencing Overall Survival and Event-Free Survival of Patients with Cervical Cancer: A Systematic Review and Meta-Analysis
- PMID: 35260545
- PMCID: PMC8919681
- DOI: 10.12659/MSM.934588
Prognostic Significance of Clinicopathological Factors Influencing Overall Survival and Event-Free Survival of Patients with Cervical Cancer: A Systematic Review and Meta-Analysis
Abstract
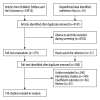
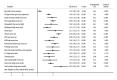
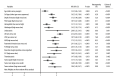
BACKGROUND Cervical cancer (CC) is the most frequent type of cancer among women and its poor prognosis is a main concern, while the prognostic factors for CC have still remained controversial. We conducted this systematic review and meta-analysis to identify the prognostic significance of clinicopathological factors, influencing overall survival (OS), and event-free survival (EFS) of CC patients. MATERIAL AND METHODS The electronic databases of PubMed, EmBase, and the Cochrane library were systematically searched for identification of eligible studies published until June 2021. The pooled hazard ratio (HR) with 95% confidence interval (CI) were calculated using the random-effects model. Sensitivity and subgroup analyses and assessment of publication bias were also conducted. RESULTS We selected 140 studies that involved 47 965 patients for the meta-analysis. The results revealed that age, cell type, depth of tumor invasion, the International Federation of Gynecology and Obstetrics stage, hemoglobin level, histological grade, leukocytosis, lymph node involvement, lymph-vascular space invasion, neutrophil-to-lymphocyte ratio, parametrial invasion, platelet-to-lymphocyte ratio, resection margin, squamous cell carcinoma antigen level, thrombocytosis, tumor grade, tumor size, and tumor volume were clinicopathological factors influencing OS and EFS of CC patients (P<0.05). CONCLUSIONS This study comprehensively identified the prognostic significance of clinicopathological factors, influencing OS, and EFS of CC patients. However, further large-scale prospective studies should be conducted to verify our findings and develop more accurate prognostic models for CC.
Conflict of interest statement
Figures
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71:209–49. - PubMed
-
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340–48. - PubMed