Reconstitution of prenyltransferase activity on nanodiscs by components of the rubber synthesis machinery of the Para rubber tree and guayule
- PMID: 35260628
- PMCID: PMC8904820
- DOI: 10.1038/s41598-022-07564-y
Reconstitution of prenyltransferase activity on nanodiscs by components of the rubber synthesis machinery of the Para rubber tree and guayule
Abstract
Natural rubber of the Para rubber tree (Hevea brasiliensis) is synthesized as a result of prenyltransferase activity. The proteins HRT1, HRT2, and HRBP have been identified as candidate components of the rubber biosynthetic machinery. To clarify the contribution of these proteins to prenyltransferase activity, we established a cell-free translation system for nanodisc-based protein reconstitution and measured the enzyme activity of the protein-nanodisc complexes. Co-expression of HRT1 and HRBP in the presence of nanodiscs yielded marked polyisoprene synthesis activity. By contrast, neither HRT1, HRT2, or HRBP alone nor a complex of HRT2 and HRBP manifested such activity. Similar analysis of guayule (Parthenium argentatum) proteins revealed that three HRT1 homologs (PaCPT1-3) manifested prenyltransferase activity only when co-expressed with PaCBP, the homolog of HRBP. Our results thus indicate that two heterologous subunits form the core prenyltransferase of the rubber biosynthetic machinery. A recently developed structure modeling program predicted the structure of such heterodimer complexes including HRT1/HRBP and PaCPT2/PaCBP. HRT and PaCPT proteins were also found to possess affinity for a lipid membrane in the absence of HRBP or PaCBP, and structure modeling implicated an amphipathic α-helical domain of HRT1 and PaCPT2 in membrane binding of these proteins.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


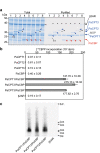


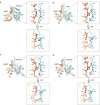
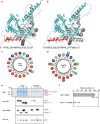
Similar articles
-
Molecular Studies of the Protein Complexes Involving Cis-Prenyltransferase in Guayule (Parthenium argentatum), an Alternative Rubber-Producing Plant.Front Plant Sci. 2019 Feb 25;10:165. doi: 10.3389/fpls.2019.00165. eCollection 2019. Front Plant Sci. 2019. PMID: 30858856 Free PMC article.
-
Identification and reconstitution of the rubber biosynthetic machinery on rubber particles from Hevea brasiliensis.Elife. 2016 Oct 28;5:e19022. doi: 10.7554/eLife.19022. Elife. 2016. PMID: 27790974 Free PMC article.
-
Subcellular localization and interactions among rubber particle proteins from Hevea brasiliensis.J Exp Bot. 2017 Nov 2;68(18):5045-5055. doi: 10.1093/jxb/erx331. J Exp Bot. 2017. PMID: 29036360 Free PMC article.
-
Biosynthesis of Natural Rubber: Current State and Perspectives.Int J Mol Sci. 2018 Dec 22;20(1):50. doi: 10.3390/ijms20010050. Int J Mol Sci. 2018. PMID: 30583567 Free PMC article. Review.
-
Guayule and Russian dandelion as alternative sources of natural rubber.Crit Rev Biotechnol. 2007 Oct-Dec;27(4):217-31. doi: 10.1080/07388550701775927. Crit Rev Biotechnol. 2007. PMID: 18085463 Review.
Cited by
-
Roles of rubber elongation factor and small rubber particle protein in rubber particles.Plant Mol Biol. 2025 May 31;115(3):71. doi: 10.1007/s11103-025-01593-7. Plant Mol Biol. 2025. PMID: 40450173 Review.
-
Transfer mechanism of cell-free synthesized membrane proteins into mammalian cells.Front Bioeng Biotechnol. 2022 Jul 22;10:906295. doi: 10.3389/fbioe.2022.906295. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35935506 Free PMC article.
-
Proteomic and Targeted Lipidomic Analyses of Fluid and Rigid Rubber Particle Membrane Domains in Guayule.Plants (Basel). 2024 Oct 24;13(21):2970. doi: 10.3390/plants13212970. Plants (Basel). 2024. PMID: 39519889 Free PMC article.
-
Half a Century of Progress: The Evolution of Wheat Germ-Based In Vitro Translation into a Versatile Protein Production Method.Int J Mol Sci. 2025 Apr 10;26(8):3577. doi: 10.3390/ijms26083577. Int J Mol Sci. 2025. PMID: 40332070 Free PMC article. Review.
-
Nanodiscs for the study of membrane proteins.Curr Opin Struct Biol. 2024 Aug;87:102844. doi: 10.1016/j.sbi.2024.102844. Epub 2024 May 24. Curr Opin Struct Biol. 2024. PMID: 38795563 Free PMC article. Review.
References
-
- Asawatreratanakul K, et al. Molecular cloning, expression and characterization of cDNA encoding cis-prenyltransferases from Hevea brasiliensis: a key factor participating in natural rubber biosynthesis. Eur. J. Biochem. 2003;270:4671–4680. - PubMed
-
- Spanò D, et al. Euphorbia characias latex: micromorphology of rubber particles and rubber transferase activity. Plant Physiol. Biochem. 2015;87:26–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

