A multicenter evaluation of computable phenotyping approaches for SARS-CoV-2 infection and COVID-19 hospitalizations
- PMID: 35260762
- PMCID: PMC8904579
- DOI: 10.1038/s41746-022-00570-4
A multicenter evaluation of computable phenotyping approaches for SARS-CoV-2 infection and COVID-19 hospitalizations
Abstract
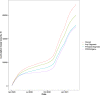
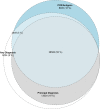
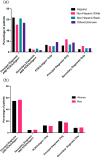
Diagnosis codes are used to study SARS-CoV2 infections and COVID-19 hospitalizations in administrative and electronic health record (EHR) data. Using EHR data (April 2020-March 2021) at the Yale-New Haven Health System and the three hospital systems of the Mayo Clinic, computable phenotype definitions based on ICD-10 diagnosis of COVID-19 (U07.1) were evaluated against positive SARS-CoV-2 PCR or antigen tests. We included 69,423 patients at Yale and 75,748 at Mayo Clinic with either a diagnosis code or a positive SARS-CoV-2 test. The precision and recall of a COVID-19 diagnosis for a positive test were 68.8% and 83.3%, respectively, at Yale, with higher precision (95%) and lower recall (63.5%) at Mayo Clinic, varying between 59.2% in Rochester to 97.3% in Arizona. For hospitalizations with a principal COVID-19 diagnosis, 94.8% at Yale and 80.5% at Mayo Clinic had an associated positive laboratory test, with secondary diagnosis of COVID-19 identifying additional patients. These patients had a twofold higher inhospital mortality than based on principal diagnosis. Standardization of coding practices is needed before the use of diagnosis codes in clinical research and epidemiological surveillance of COVID-19.
© 2022. The Author(s).
Conflict of interest statement
R.K. is the founder of Evidence2Health, a digital precision healthcare platform. H.M.K. works under contract with the Centers for Medicare & Medicaid Services to support quality measurement programs, was a recipient of a research grant from Johnson & Johnson, through Yale University, to support clinical trial data sharing; was a recipient of a research agreement, through Yale University, from the Shenzhen Center for Health Information for work to advance intelligent disease prevention and health promotion; collaborates with the National Center for Cardiovascular Diseases in Beijing; receives payment from the Arnold & Porter Law Firm for work related to the Sanofi clopidogrel litigation, from the Martin Baughman Law Firm for work related to the Cook Celect IVC filter litigation, and from the Siegfried and Jensen Law Firm for work related to Vioxx litigation; chairs a Cardiac Scientific Advisory Board for UnitedHealth; was a member of the IBM Watson Health Life Sciences Board; is a member of the Advisory Board for Element Science, the Advisory Board for Facebook, and the Physician Advisory Board for Aetna; and is the co-founder of Hugo Health, a personal health information platform, and co-founder of Refactor Health, a healthcare AI-augmented data management company. W.L.S. was an investigator for a research agreement, through Yale University, from the Shenzhen Center for Health Information for work to advance intelligent disease prevention and health promotion; collaborates with the National Center for Cardiovascular Diseases in Beijing; is a technical consultant to Hugo Health, a personal health information platform, and co-founder of Refactor Health, an AI-augmented data management platform for healthcare; is a consultant for Interpace Diagnostics Group, a molecular diagnostics company. J.S.R. currently receives research support through Yale University from Johnson and Johnson to develop methods of clinical trial data sharing, from the Medical Device Innovation Consortium as part of the National Evaluation System for Health Technology (NEST), from the Food and Drug Administration for the Yale-Mayo Clinic Center for Excellence in Regulatory Science and Innovation (CERSI) program (U01FD005938); from the Agency for Healthcare Research and Quality (R01HS022882), from the National Heart, Lung and Blood Institute of the National Institutes of Health (NIH) (R01HS025164, R01HL144644), and from the Laura and John Arnold Foundation to establish the Good Pharma Scorecard at Bioethics International. In the past 36 months, NDS has received research support through Mayo Clinic from the Food and Drug Administration to establish Yale-Mayo Clinic Center for Excellence in Regulatory Science and Innovation (CERSI) program (U01FD005938); the Centers of Medicare and Medicaid Innovation under the Transforming Clinical Practice Initiative (TCPI); the Agency for Healthcare Research and Quality (R01HS025164; R01HS025402; R03HS025517; K12HS026379); the National Heart, Lung and Blood Institute of the National Institutes of Health (NIH) (R56HL130496; R01HL131535; R01HL151662); the National Science Foundation; from the Medical Device Innovation Consortium as part of the National Evaluation System for Health Technology (NEST) and the Patient Centered Outcomes Research Institute (PCORI) to develop a Clinical Data Research Network (LHSNet). E.S.T. serves on the advisory board of Roche Diagnostics and Serimmune. R.M. serves on the data safety and monitoring board for a phase 1 trial of a COVID therapeutic being investigated by Noveome. D.R.P. serves on the advisory board of Tangen Biosciences and is a co-founder of M/Z Diagnostics. The other authors do not report any relevant disclosures.
Figures


Update of
-
Accuracy of Computable Phenotyping Approaches for SARS-CoV-2 Infection and COVID-19 Hospitalizations from the Electronic Health Record.medRxiv [Preprint]. 2021 May 13:2021.03.16.21253770. doi: 10.1101/2021.03.16.21253770. medRxiv. 2021. Update in: NPJ Digit Med. 2022 Mar 8;5(1):27. doi: 10.1038/s41746-022-00570-4. PMID: 34013299 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

