S-SCAM inhibits Axin-dependent synaptic function of GSK3β in a sex-dependent manner
- PMID: 35260764
- PMCID: PMC8904762
- DOI: 10.1038/s41598-022-08220-1
S-SCAM inhibits Axin-dependent synaptic function of GSK3β in a sex-dependent manner
Abstract
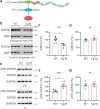
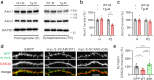
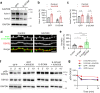
S-SCAM/MAGI-2 gene duplication is associated with schizophrenia (SCZ). S-SCAM overexpression in the forebrain induces SCZ-like phenotypes in a transgenic (Tg) mouse model. Interestingly, S-SCAM Tg mice show male-specific impairments in synaptic plasticity and working memory. However, mechanisms underlying the sex-specific deficits remain unknown. Here we report that S-SCAM Tg mice have male-specific deficits in synaptic GSK3β functions, as shown by reduced synaptic protein levels and increased inhibitory phosphorylation of GSK3β. This GSK3β hyper-phosphorylation was associated with increased CaMKII activities. Notably, synaptic levels of Axin1, to which GSK3β binds in competition with S-SCAM, were also reduced in male S-SCAM Tg mice. We demonstrated that Axin-binding is required for the S-SCAM overexpression-induced synaptic GSK3β reduction. Axin stabilization using XAV939 rescued the GSK3β deficits and restored the temporal activation of GSK3β during long-term depression in S-SCAM overexpressing neurons. Interestingly, synaptic Axin2 levels were increased in female S-SCAM Tg mice. Female sex hormone 17β-estradiol increased Axin2 expression and increased synaptic GSK3β levels in S-SCAM overexpressing neurons. These results reveal the role of S-SCAM in controlling Axin-dependent synaptic localization of GSK3β. Moreover, our studies point out the pathological relevance of GSK3β hypofunction found in humans and contribute to understanding the molecular underpinnings of sex differences in SCZ.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Sumita K, et al. Synaptic scaffolding molecule (S-SCAM) membrane-associated guanylate kinase with inverted organization (MAGI)-2 is associated with cell adhesion molecules at inhibitory synapses in rat hippocampal neurons. J. Neurochem. 2007;100:154–166. doi: 10.1111/j.1471-4159.2006.04170.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

