Ancient and recent differences in the intrinsic susceptibility of Mycobacterium tuberculosis complex to pretomanid
- PMID: 35260883
- PMCID: PMC9155602
- DOI: 10.1093/jac/dkac070
Ancient and recent differences in the intrinsic susceptibility of Mycobacterium tuberculosis complex to pretomanid
Abstract
Objectives: To develop a robust phenotypic antimicrobial susceptibility testing (AST) method with a correctly set breakpoint for pretomanid (Pa), the most recently approved anti-tuberculosis drug.
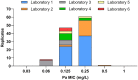
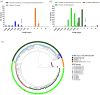
Methods: The Becton Dickinson Mycobacterial Growth Indicator Tube™ (MGIT) system was used at six laboratories to determine the MICs of a phylogenetically diverse collection of 356 Mycobacterium tuberculosis complex (MTBC) strains to establish the epidemiological cut-off value for pretomanid. MICs were correlated with WGS data to study the genetic basis of differences in the susceptibility to pretomanid.
Results: We observed ancient differences in the susceptibility to pretomanid among various members of MTBC. Most notably, lineage 1 of M. tuberculosis, which is estimated to account for 28% of tuberculosis cases globally, was less susceptible than lineages 2, 3, 4 and 7 of M. tuberculosis, resulting in a 99th percentile of 2 mg/L for lineage 1 compared with 0.5 mg/L for the remaining M. tuberculosis lineages. Moreover, we observed that higher MICs (≥8 mg/L), which probably confer resistance, had recently evolved independently in six different M. tuberculosis strains. Unlike the aforementioned ancient differences in susceptibility, these recent differences were likely caused by mutations in the known pretomanid resistance genes.
Conclusions: In light of these findings, the provisional critical concentration of 1 mg/L for MGIT set by EMA must be re-evaluated. More broadly, these findings underline the importance of considering the global diversity of MTBC during clinical development of drugs and when defining breakpoints for AST.
© The Author(s) 2022. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures
References
-
- World Health Organization . Global tuberculosis report 2020. https://apps.who.int/iris/handle/10665/336069.
-
- European Medicines Agency . Dovprela: EPAR – product information. https://www.ema.europa.eu/en/documents/product-information/dovprela-epar....
-
- World Health Organization . Meeting report of the WHO expert consultation on the definition of extensively drug-resistant tuberculosis. 27–29 October 2020. https://apps.who.int/iris/handle/10665/338776.
-
- Conradie F, Everitt D, Olugbosi Met al. High rate of successful outcomes treating highly resistant TB in the ZeNix study of pretomanid, bedaquiline and alternative doses and durations of linezolid. 11th IAS Conference on HIV Science, 2021. Late breaker OALB01LB02.
Publication types
MeSH terms
Substances
Grants and funding
- Australia's Department of Foreign Affairs and Trade
- Germany's Federal Ministry of Education and Research
- Irish Aid
- Netherlands Ministry of Foreign Affairs
- United Kingdom Department of Health
- United Kingdom Foreign
- Commonwealth and Development Office
- United States Agency for International Development
- South African Medical Research Council
- Tuberculosis Omics Research Consortium
- G0F8316N/FWO
- GATES/Bill & Melinda Gates Foundation/United States
- Federal Ministry of Education and Research
- Ministry of Foreign Affairs
- Foreign, Commonwealth and Development Office
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

