Severe asthma in children: Description of a large multidisciplinary clinical cohort
- PMID: 35261210
- PMCID: PMC9119906
- DOI: 10.1002/ppul.25887
Severe asthma in children: Description of a large multidisciplinary clinical cohort
Abstract
Background: Children with severe asthma have substantial morbidity and healthcare utilization. Pediatric severe asthma is a heterogeneous disease, and a multidisciplinary approach can improve the diagnosis and management of these children.
Methods: We reviewed the electronic health records for patients seen in the Severe Asthma Clinic (SAC) at UPMC Children's Hospital of Pittsburgh between August 2012 and October 2019.
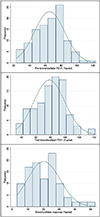
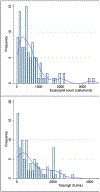
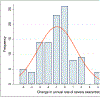
Results: Of the 110 patients in whom we extracted data, 46% were female, 48% were Black/African American, and 41% had ≥1 admission to the pediatric intensive care unit (PICU) for asthma. Compared to patients without a PICU admission, those with ≥1 PICU admission were more likely to be non-White (64.4% vs. 41.5%, p = 0.031) and more atopic (eosinophil count geometric mean = 673 vs. 319 cells/mm3 , p = 0.002; total IgE geometric mean = 754 vs. 303 KU/L, p = 0.003), and to have lower pre-bronchodilator FEV1 (58.6% [±18.1%] vs. 69.9% [±18.7%], p = 0.002) and elevated FeNO (60% vs. 22%, p = 0.02). In this cohort, 84% of patients were prescribed high-dose ICS/LABA and 36% were on biologics. Following enrollment in the SAC, severe exacerbations decreased from 3.2/year to 2.2/year (p < 0.0001); compared to the year before joining the SAC, in the following year the group had 106 fewer severe exacerbations.
Conclusions: This large cohort of children with severe asthma had a high level of morbidity and healthcare utilization. Patients with a history of PICU admissions for asthma were more likely to be nonwhite and highly atopic, and to have lower lung function. Our data support a positive impact of a multidisciplinary clinic on patients with severe childhood asthma.
Keywords: multidisciplinary clinic; severe asthma; severe childhood asthma.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
Figures