The ecology and evolution of the monito del monte, a relict species from the southern South America temperate forests
- PMID: 35261741
- PMCID: PMC8888251
- DOI: 10.1002/ece3.8645
The ecology and evolution of the monito del monte, a relict species from the southern South America temperate forests
Abstract
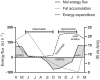
The arboreal marsupial monito del monte (genus Dromiciops, with two recognized species) is a paradigmatic mammal. It is the sole living representative of the order Microbiotheria, the ancestor lineage of Australian marsupials. Also, this marsupial is the unique frugivorous mammal in the temperate rainforest, being the main seed disperser of several endemic plants of this ecosystem, thus acting as keystone species. Dromiciops is also one of the few hibernating mammals in South America, spending half of the year in a physiological dormancy where metabolism is reduced to 10% of normal levels. This capacity to reduce energy expenditure in winter contrasts with the enormous energy turnover rate they experience in spring and summer. The unique life history strategies of this living Microbiotheria, characterized by an alternation of life in the slow and fast lanes, putatively represent ancestral traits that permitted these cold-adapted mammals to survive in this environment. Here, we describe the ecological role of this emblematic marsupial, summarizing the ecophysiology of hibernation and sociality, updated phylogeographic relationships, reproductive cycle, trophic relationships, mutualisms, conservation, and threats. This marsupial shows high densities, despite presenting slow reproductive rates, a paradox explained by the unique characteristics of its three-dimensional habitat. We finally suggest immediate actions to protect these species that may be threatened in the near future due to habitat destruction and climate change.
Keywords: Australidelphia; climate change; conservation; hibernation; marsupial; seed dispersal.
© 2022 The Authors. Ecology and Evolution published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures







References
-
- Aizen, M. A. (2003). Influences of animal pollination and seed dispersal on winter flowering in a temperate mistletoe. Ecology, 84(10), 2613–2627. 10.1890/02-0521 - DOI
-
- Amico, G. C. , Rodríguez‐Cabal, M. A. , & Aizen, M. A. (2009). The potential key seed‐dispersing role of the arboreal marsupial Dromiciops gliroides . Acta Oecologica‐International Journal of Ecology, 35(1), 8–13. 10.1016/j.actao.2008.07.003 - DOI
-
- Amico, G. C. , Rodríguez‐Cabal, M. A. , & Aizen, M. A. (2011). Geographic variation in fruit colour is associated with contrasting seed disperser assemblages in a south‐Andean mistletoe. Ecography, 34(2), 318–326. 10.1111/j.1600-0587.2010.06459.x - DOI
-
- Amico, G. C. , Sasal, Y. , Vidal‐Russell, R. , Aizen, M. A. , & Morales, J. M. (2017). Consequences of disperser behaviour for seedling establishment of a mistletoe species. Austral Ecology, 42(8), 900–907. 10.1111/aec.12517 - DOI
Publication types
LinkOut - more resources
Full Text Sources

