The histopathological spectrum of kidney biopsies in patients with thymoma and myasthenia gravis: a report of 24 biopsies from a single institution
- PMID: 35261763
- PMCID: PMC8894933
- DOI: 10.1093/ckj/sfaa276
The histopathological spectrum of kidney biopsies in patients with thymoma and myasthenia gravis: a report of 24 biopsies from a single institution
Abstract
Background: Nephropathy in patients with thymic diseases such as thymoma and myasthenia gravis (MG) is rare and has been described mostly as isolated case reports. Here we evaluate a series of kidney biopsies from patients with thymoma and/or MG from a single institution in order to better define the spectrum and relative frequencies of thymic disease-associated nephropathies.
Methods: We conducted a retrospective case series study of 32 462 native kidney biopsies from January 2005 through December 2019 at Cedars-Sinai Medical Center, Los Angeles, CA, USA.
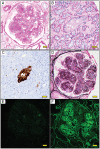
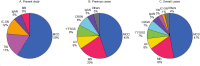
Results: Twenty-four biopsy specimens (0.07%) from patients with a history of thymoma and/or MG were identified. Two patients had repeat biopsies. The most common pathologic diagnosis that could be immunologically attributed to thymic disease was minimal change disease (MCD; 45%), followed by tubulointerstitial nephritis (TIN; 14%), immune complex (IC)-mediated glomerulonephritis (9%), membranous nephropathy (5%) and immunoglobulin A (IgA) nephropathy (5%). Interestingly, 50% of the MCD and 67% of TIN cases concomitantly showed mild IgG-dominant IC deposition in mesangial areas and/or in tubular basement membranes. In the two patients with repeat biopsies, mild mesangial IC deposition developed in the MCD patient but disappeared in the TIN patient with the second biopsy. Pathologic diagnoses unlikely related to the underlying thymic disease were diabetic glomerulosclerosis (9%), acute tubular necrosis (9%) and monoclonal Ig deposition disease (5%).
Conclusions: Thymic disease is associated with a wide spectrum of kidney diseases affecting the glomerular and tubulointerstitial compartments, often with low-grade IC deposition. These findings suggest a role of immunologic dysregulation in the pathogenesis of thymic disease-associated nephropathy.
Keywords: immune complex deposition; minimal change disease; onconephrology; paraneoplastic syndrome; thymus; tubulointerstitial nephritis.
© The Author(s) 2021. Published by Oxford University Press on behalf of ERA-EDTA.
Figures



Similar articles
-
Renal and thymic pathology in thymoma-associated nephropathy: report of 21 cases and review of the literature.Nephrol Dial Transplant. 2005 Jun;20(6):1075-82. doi: 10.1093/ndt/gfh615. Epub 2005 Mar 23. Nephrol Dial Transplant. 2005. PMID: 15788438 Review.
-
The histopathologic spectrum of kidney biopsies in patients with inflammatory bowel disease.Clin J Am Soc Nephrol. 2014 Feb;9(2):265-70. doi: 10.2215/CJN.04660513. Epub 2013 Nov 21. Clin J Am Soc Nephrol. 2014. PMID: 24262508 Free PMC article.
-
The Czech registry of renal biopsies. Occurrence of renal diseases in the years 1994-2000.Nephrol Dial Transplant. 2004 Dec;19(12):3040-9. doi: 10.1093/ndt/gfh521. Epub 2004 Oct 26. Nephrol Dial Transplant. 2004. PMID: 15507479
-
C1q nephropathy: a variant of focal segmental glomerulosclerosis.Kidney Int. 2003 Oct;64(4):1232-40. doi: 10.1046/j.1523-1755.2003.00218.x. Kidney Int. 2003. PMID: 12969141
-
Pan-nephritis (glomerulonephritis, arteriolitis, and tubulointerstitial nephritis) associated with predominant mesangial C1q deposition and hypocomplementemia: a variant type of C1q nephropathy?Am J Kidney Dis. 1996 Apr;27(4):583-7. doi: 10.1016/s0272-6386(96)90171-7. Am J Kidney Dis. 1996. PMID: 8678071 Review.
Cited by
-
Multi-organ single-cell transcriptomics of immune cells uncovered organ-specific gene expression and functions.Sci Data. 2024 Mar 27;11(1):316. doi: 10.1038/s41597-024-03152-z. Sci Data. 2024. PMID: 38538617 Free PMC article.
References
-
- Bernard C, Frih H, Pasquet F et al. Thymoma associated with autoimmune diseases: 85 cases and literature review. Autoimmun Rev 2016; 15: 82–92 - PubMed
-
- Souadjian JV, Enriquez P, Silverstein MN et al. The spectrum of diseases associated with thymoma. Coincidence or syndrome? Arch Intern Med 1974; 134: 374–379 - PubMed
-
- Fujii Y. Thymus, thymoma and myasthenia gravis. Surg Today 2013; 43: 461–466 - PubMed
-
- Jhaveri KD, Shah HH, Calderon K et al. Glomerular diseases seen with cancer and chemotherapy: a narrative review. Kidney Int 2013; 84: 34–44 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

