Cisplatin plus anti-PD-1 antibody enhanced treatment efficacy in advanced esophageal squamous cell carcinoma
- PMID: 35261780
- PMCID: PMC8899995
Cisplatin plus anti-PD-1 antibody enhanced treatment efficacy in advanced esophageal squamous cell carcinoma
Abstract
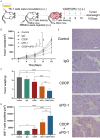
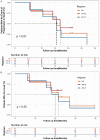
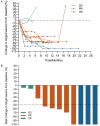
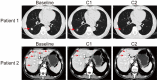
Therapies for patients with advanced esophageal squamous cell carcinoma (ESCC) are limited and accompanied by dismal prognosis. Here we use ESCC cell line K30 and TE-1 to investigate the antitumor efficacy of cisplatin plus anti-PD-1 antibody. Enhanced antitumor effects and increased CD8+ tumor-infiltrating lymphocytes of combination therapy were observed in TE-1 cells bearing humanized mice model. Lower cell viability and more cell apoptosis were found in the combination therapy in vitro. We next analyzed clinical data from patients with advanced ESCC received cisplatin-based chemotherapy plus an anti-PD-1 antibody (Tislelizumab or Sintilimab) as first line therapy from two clinical trials (NCT03469557, NCT03748134). With the response rate of 81.8%, duration of response of 15.2 months, median progression-free survival of 15.5 months, median overall survival of 21.5 months and manageable toxicity in patients with advanced ESCC, we demonstrated that cisplatin-based chemotherapy plus anti-PD-1 antibody is an effective and safe option. We further confirmed sublethal cisplatin could induce PD-L1 expression in ESCC cells and cisplatin-treated ESCC cells suppressed the activation and function of immune cells while the addition of sintilimab prevented this process. These results highlight the effectiveness of cisplatin combining with anti-PD-1 antibody in patients with advanced ESCC, revealed its capability to promote the PD-L1 expression in ESCC cells and act synergistically with anti-PD-1 antibody to restore exhausted immune cells activities, thus providing a theoretical basis for further explorations in the mechanism of the combination treatment of cisplatin-based chemotherapy with immune checkpoint inhibitors in ESCC.
Keywords: Esophageal squamous cell carcinoma; PD-1; chemotherapy; cisplatin; immunotherapy.
AJCR Copyright © 2022.
Conflict of interest statement
None.
Figures







Similar articles
-
RATIONALE 311: tislelizumab plus concurrent chemoradiotherapy for localized esophageal squamous cell carcinoma.Future Oncol. 2021 Nov;17(31):4081-4089. doi: 10.2217/fon-2021-0632. Epub 2021 Jul 16. Future Oncol. 2021. PMID: 34269067 Clinical Trial.
-
Safety and Feasibility of Radiotherapy Plus Camrelizumab for Locally Advanced Esophageal Squamous Cell Carcinoma.Oncologist. 2021 Jul;26(7):e1110-e1124. doi: 10.1002/onco.13797. Epub 2021 Jun 5. Oncologist. 2021. PMID: 33893689 Free PMC article. Clinical Trial.
-
Tislelizumab Plus Chemotherapy as First-line Treatment for Advanced Esophageal Squamous Cell Carcinoma and Gastric/Gastroesophageal Junction Adenocarcinoma.Clin Cancer Res. 2020 Sep 1;26(17):4542-4550. doi: 10.1158/1078-0432.CCR-19-3561. Epub 2020 Jun 19. Clin Cancer Res. 2020. PMID: 32561664 Clinical Trial.
-
PD-1 inhibitors in advanced esophageal squamous cell carcinoma: a survival analysis of reconstructed patient-level data.Front Pharmacol. 2024 Jul 18;15:1408458. doi: 10.3389/fphar.2024.1408458. eCollection 2024. Front Pharmacol. 2024. PMID: 39092218 Free PMC article.
-
Checkpoint inhibitors for gastroesophageal cancers: dissecting heterogeneity to better understand their role in first-line and adjuvant therapy.Ann Oncol. 2021 May;32(5):590-599. doi: 10.1016/j.annonc.2021.02.004. Epub 2021 Feb 17. Ann Oncol. 2021. PMID: 33609722 Review.
Cited by
-
Integrin-linked kinase affects the sensitivity of esophageal squamous cell carcinoma cells to chemotherapy with cisplatin via the Wnt/beta-catenin signaling pathway.Bioengineered. 2022 May;13(5):12532-12547. doi: 10.1080/21655979.2022.2076497. Bioengineered. 2022. PMID: 35587162 Free PMC article.
-
Cost-Effectiveness Analysis of Tislelizumab Plus Chemotherapy as First-Line Treatment for Advanced or Metastatic Esophageal Squamous Cell Carcinoma in China.Risk Manag Healthc Policy. 2023 Nov 15;16:2447-2458. doi: 10.2147/RMHP.S436750. eCollection 2023. Risk Manag Healthc Policy. 2023. PMID: 38024498 Free PMC article.
-
Delayed vaccine-induced CD8+ T cell expansion by topoisomerase I inhibition mediates enhanced CD70-dependent tumor eradication.J Immunother Cancer. 2023 Nov 29;11(11):e007158. doi: 10.1136/jitc-2023-007158. J Immunother Cancer. 2023. PMID: 38030302 Free PMC article.
-
Sirtuin1 (sirt1) regulates the glycolysis pathway and decreases cisplatin chemotherapeutic sensitivity to esophageal squamous cell carcinoma.Cancer Biol Ther. 2024 Dec 31;25(1):2365449. doi: 10.1080/15384047.2024.2365449. Epub 2024 Jun 12. Cancer Biol Ther. 2024. PMID: 38865161 Free PMC article.
-
Genetic polymorphisms as potential pharmacogenetic biomarkers for platinum-based chemotherapy in non-small cell lung cancer.Mol Biol Rep. 2024 Jan 13;51(1):102. doi: 10.1007/s11033-023-08915-2. Mol Biol Rep. 2024. PMID: 38217759 Review.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381–387. - PubMed
-
- Edgren G, Adami HO, Vainio EW, Nyrén O. A global assessment of the oesophageal adenocarcinoma epidemic. Gut. 2013;62:1406–1414. - PubMed
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
