Recombinant cell-detecting RaDR-GFP in mice reveals an association between genomic instability and radiation-induced-thymic lymphoma
- PMID: 35261787
- PMCID: PMC8899999
Recombinant cell-detecting RaDR-GFP in mice reveals an association between genomic instability and radiation-induced-thymic lymphoma
Abstract
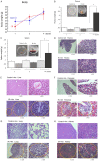
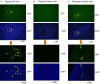
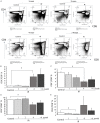
In this study, we aimed to investigate how homologous recombinant (HR)-related genomic instability is involved in ionizing radiation (IR)-induced thymic lymphoma in mice. We divided five-week-old Rosa26 Direct Repeat-GFP (RaDR-GFP) transgenic mice into non-IR control and IR groups and exposed the mice in the IR group to a 7.2 Gy dose of γ-rays, delivered in 1.8 Gy fractions, once a week for four weeks. We then estimated mouse survival and recorded their body, thymus, and spleen weights. The frequency of HR events in the chromosomes of the thymus, bone marrow, and spleen cells and the phenotype of thymic lymphoma cells were analyzed using fluorescence-activated cell sorting (FACS). We found that most mice in the IR group developed thymic lymphoma, their survival rate decreasing to 20% after 180 days of IR exposure, whereas no mice died in the non-IR control group until day 400. The thymus and spleen weighed significantly more in the IR-4-month group than that in the non-IR group; however, we observed no significant differences between the body weights of the control and IR mice. FACS analysis indicated that the frequency of HR events significantly increased at two and four months after the last IR dose in the bone marrow and thymus cells, but not in the spleen cells of RaDR-GFP transgenic mice, suggesting that recombinant cells accumulated in the thymus upon IR exposure. This suggests that IR induces genome instability, revealed as increased HR, that drives the development of thymic lymphoma. Additionally, phenotypic analysis of lymphoma cells showed an increase in the CD4-/CD8+ (CD8SP) cell population and a decrease in the CD4+/CD8- (CD4SP) cell population in the IR-4-month group compared to that in the non-IR group, indicating that IR induces an aberrant cell phenotype characteristic of lymphoma. In conclusion, we observed a significant increase in HR events and abnormal phenotype in thymic lymphoma cells at two and four months after IR exposure in both the thymus and bone marrow tissues, suggesting that genomic instability is involved in the early stages of thymic lymphomagenesis. Our study indicates that HR-visualizing RaDR-GFP transgenic mice can help explore the links between the molecular mechanisms of genome instability and IR-induced tumorigenesis.
Keywords: Radiation; genome instability; homologous recombination (replication stress); lymphoma.
AJCR Copyright © 2022.
Conflict of interest statement
None.
Figures


Similar articles
-
Reduction of Delayed Homologous Recombination by Induction of Radioadaptive Response in RaDR-GFP Mice (Yonezawa Effect): An Old Player With a New Role.Dose Response. 2019 Mar 4;17(1):1559325819833840. doi: 10.1177/1559325819833840. eCollection 2019 Jan-Mar. Dose Response. 2019. PMID: 30858771 Free PMC article.
-
Rosa26-GFP direct repeat (RaDR-GFP) mice reveal tissue- and age-dependence of homologous recombination in mammals in vivo.PLoS Genet. 2014 Jun 5;10(6):e1004299. doi: 10.1371/journal.pgen.1004299. eCollection 2014 Jun. PLoS Genet. 2014. PMID: 24901438 Free PMC article.
-
NTP Toxicology and Carcinogenesis Studies of Phenolphthalein (CAS No. 77-09-8) in F344/N Rats and B6C3F1 Mice (Feed Studies).Natl Toxicol Program Tech Rep Ser. 1996 Nov;465:1-354. Natl Toxicol Program Tech Rep Ser. 1996. PMID: 12579199
-
Cellular events in radiation-induced lymphomagenesis.Int J Radiat Biol. 1990 Apr;57(4):693-8. doi: 10.1080/09553009014550861. Int J Radiat Biol. 1990. PMID: 1969901 Review.
-
Transgenic mice harboring direct repeat substrates reveal key underlying causes of homologous recombination in vivo.DNA Repair (Amst). 2022 Dec;120:103419. doi: 10.1016/j.dnarep.2022.103419. Epub 2022 Oct 10. DNA Repair (Amst). 2022. PMID: 36257175 Free PMC article. Review.
References
-
- Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008;18:134–47. - PubMed
-
- Vignard J, Mirey G, Salles B. Ionizing-radiation induced DNA double-strand breaks: a direct and indirect lighting up. Radiother Oncol. 2013;108:362–9. - PubMed
-
- Santivasi WL, Xia F. Ionizing radiation-induced DNA damage, response, and repair. Antioxid Redox Signal. 2014;21:251–9. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
