Glycolysis in the progression of pancreatic cancer
- PMID: 35261808
- PMCID: PMC8900001
Glycolysis in the progression of pancreatic cancer
Abstract
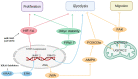
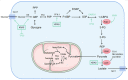
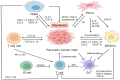
Metabolic reprogramming, as a key hallmark of cancers, leads to the malignant behavior of pancreatic cancer, which is closely related to tumor development and progression, as well as the supportive tumor microenvironments. Although cells produce adenosine triphosphate (ATP) from glucose by glycolysis when lacking oxygen, pancreatic cancer cells elicit metabolic conversion from oxide phosphorylation to glycolysis, which is well-known as "Warburg effect". Glycolysis is critical for cancer cells to maintain their robust biosynthesis and energy requirement, and it could promote tumor initiation, invasion, angiogenesis, and metastasis to distant organs. Multiple pathways are involved in the alternation of glycolysis for pancreatic cancer cells, including UHRF1/SIRT4 axis, PRMT5/FBW7/cMyc axis, JWA/AMPK/FOXO3a/FAK axis, KRAS/TP53/TIGAR axis, etc. These signaling pathways play an important role in glycolysis and are potential targets for the treatment of pancreatic cancer. Mutations in glycolytic enzymes (such as LDH, PKM2, and PGK1) also contribute to the early diagnosis and monitoring of pancreatic cancer. In this review, we summarized the recent advances on the mechanisms for glycolysis in pancreatic cancer and the function of glycolysis in the progression of pancreatic cancer, which suggested new targets for cancer diagnosis and treatment.
Keywords: Pancreatic cancer; glycolysis; metabolism; tumor microenvironment; tumor progression.
AJCR Copyright © 2022.
Conflict of interest statement
None.
Figures
References
-
- Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. - PubMed
-
- Chen BB, Tien YW, Chang MC, Cheng MF, Chang YT, Wu CH, Chen XJ, Kuo TC, Yang SH, Shih IL, Lai HS, Shih TT. PET/MRI in pancreatic and periampullary cancer: correlating diffusion-weighted imaging, MR spectroscopy and glucose metabolic activity with clinical stage and prognosis. Eur J Nucl Med Mol Imaging. 2016;43:1753–1764. - PubMed
-
- Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
