Comparison of two nucleic acid amplification tests (NAATs) and two antigen tests for detection of SARS-CoV-2 from upper respiratory specimens
- PMID: 35261999
- PMCID: PMC8019354
- DOI: 10.1016/j.jcvp.2021.100011
Comparison of two nucleic acid amplification tests (NAATs) and two antigen tests for detection of SARS-CoV-2 from upper respiratory specimens
Abstract
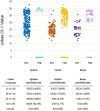
There are numerous tests available for acute diagnosis of SARS-CoV-2, the virus that causes the disease COVID-19. These tests fall into two main groups: nucleic acid amplification tests (NAATs) and antigen-based assays. We evaluated the clinical performance of two rapid antigen assays (BD Veritor System for Rapid Detection of SARS CoV-2 and Abbott BinaxNOW COVID-19 Ag Card) and one NAAT (Hologic Aptima SARS CoV-2 Assay) by comparing them with the initial test of record, the Roche cobas SARS-CoV-2 assay; the antigen tests were also compared to Aptima. We tested remnant frozen specimens from patients suspected of SARS-CoV-2 infections (either due to symptoms or exposure) on the comparator platforms to evaluate assay performance across a wide range of positive results, including cobas cycle threshold (Ct) values ranging between 12 and 35. We tested 250 previous positive and 50 previous negative specimens and found 95.6% positive percent agreement (PPA) with the Aptima assay. The few discrepancies between the NAATs occurred only when Ct values were >32. Agreement was much lower for the rapid antigen tests, with 45.2%/47.3% PPA for the Veritor and 47.0%/47.0% PPA for the Binax compared to cobas/Aptima. Discrepancies occurred when cobas Ct values were >20 for Veritor and >25 for Binax. The negative percent agreement (NPA) was 100% for all assay comparisons. These data indicate similar performance between the cobas and Aptima NAATs but demonstrate that antigen-based assays may be insufficient to diagnose SARS-CoV-2 infection when lower levels of the virus are shed.
Keywords: Antigen testing; Automation; COVID-19; Nucleic acid amplification test; SARS-CoV-2.
© 2021 The Author(s). Published by Elsevier Ltd.
Conflict of interest statement
All authors have reviewed and approved the manuscript and have no conflicts of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous
