Field performance evaluation of the PanBio rapid SARS-CoV-2 antigen assay in an epidemic driven by the B.1.351 variant in the Eastern Cape, South Africa
- PMID: 35262001
- PMCID: PMC8025602
- DOI: 10.1016/j.jcvp.2021.100013
Field performance evaluation of the PanBio rapid SARS-CoV-2 antigen assay in an epidemic driven by the B.1.351 variant in the Eastern Cape, South Africa
Abstract
Background: South Africa was the African country with the most recorded cases of SARS-CoV-2 during 2020, experiencing 2 waves of infection. During the first wave, diagnostics were largely based on reverse transcription-linked PCR (RT-PCR). The Abbott PanBio antigen test was deployed during the 2nd wave which may have been driven by emergence of the B.1.351 variant. At the time of evaluation in mid-November 2020, B.1.351 was the dominant circulating virus in Nelson Mandela Bay, in the Eastern Cape Province.
Methods: Used PanBio antigen swabs (collected from patients with genetically characterised virus) were first validated as suitable for PCR. A prospective study was then undertaken to evaluate assay performance in the field. Testing was conducted at mobile community testing centres on 677 ambulant patients. Used swabs were kept and tested by RT-PCR.
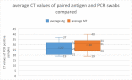
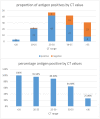
Results: During initial validation, used swabs in proprietary lysis buffer were found to be suitable for PCR and secondly, the PB assay reliably detected patients infected with B.1.351. In the field study, of 146 RT-PCR positive individuals, 101 were RTD positive in the clinic. The RTD had a sensitivity of 69.2% (95%CI 61.4, 75.8) and specificity of 99.0% (95%CI 98.8, 99.3). Sensitivity was dependent on the amount of viral RNA in clinical samples, as reflected by the PCR cycle threshold (CT) value.
Conclusions: The assay reliably detected B.1.351 infections in ambulatory ill patients during initial validation and in field testing. In the field, assay sensitivity was >90% in patients with high viral loads who are expected to be most infectious. Negative and positive predictive values were also >90%.
Keywords: 501Y.v2; 501Y.v2, 501Y variant 2; B.1.351; COVID-19; COVID-19, Coronavirus disease 2019; CT, cycle threshold; GISAID, global initiative for sharing all influenza data; IQR, interquartile range; NP, nasopharyngeal; PCR, polymerase chain reaction; Point of care; RT-PCR, reverse transcription polymerase chain reaction; RTD, rapid test device; Rapid antigen test; SARS-CoV-2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, world health organisation.
© 2021 The Author(s). Published by Elsevier Ltd.
Conflict of interest statement
The authors report no competing interests.
Figures

References
-
- SAMRC, Report on weekly deaths in South Africa, South Africa Med. Res. Counc. 2021 (2020) 1. https://www.samrc.ac.za/reports/report-weekly-deaths-south-africa.
-
- Waasila Jassat1 L.B. Cheryl Cohen2, Caroline Mudara1, The first and second wave of COVID-19 In three districts of South Africa, COVID-19 Spec. Public Heal. Surveill. Bull. COVID-19 Spec. PUBLIC Heal. Surveill. Bull. 2021;18:1–24.
-
- Cunningham J., Beese S., Dretzke J., Im H., Mj P., Im H., Mj P., Hoo L., Mmg L., Spijker R. 2020. Diagnosis of SARS-CoV-2 Infection (Review)www.cochranelibrary.com V.D.B. A. - DOI
-
- WHO, Antigen-detection in the Diagnosis of SARS-CoV-2 Infection Using Rapid Immunoassays Interim guidance, 11 September 2020, World Health Organisation (2020). https://apps.who.int/iris/handle/10665/334253.
-
- Linares M., Pérez-Tanoira R., Carrero A., Romanyk J., Pérez-García F., Gómez-Herruz P., Arroyo T., Cuadros J. Panbio antigen rapid test is reliable to diagnose SARS-CoV-2 infection in the first 7 days after the onset of symptoms. J. Clin. Virol. 2020;133:3–6. doi: 10.1016/j.jcv.2020.104659. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
