SARS-CoV-2 loads in urine, sera and stool specimens in association with clinical features of COVID-19 patients
- PMID: 35262032
- PMCID: PMC8673951
- DOI: 10.1016/j.jcvp.2021.100059
SARS-CoV-2 loads in urine, sera and stool specimens in association with clinical features of COVID-19 patients
Abstract
Background: COVID-19 pandemic continues to be a priority in public health worldwide, and factors inherent to SARS-CoV-2 pathogenesis and genomic characteristics are under study. Investigations that evaluate possible risk factors for infection, clinical manifestations, and viral shedding in different specimens also need to clarify possible associations with COVID-19 prognosis and disease outcomes.
Study design: In this study, we evaluated SARS-CoV-2 positivity and estimated viral loads by real-time RT-PCR in stool, sera, and urine samples from 35 patients, with a positive SARS-CoV-2 RNA molecular test in respiratory sample, attended at a University COVID-19 referral hospital in Goiania, Goias, Brazil. Whole-genome sequencing was also performed in samples with higher viral load.
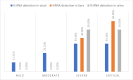
Results: The positivity index was 51.43%, 14.28%, and 5.71% in stool, sera, and urine specimens, respectively. The median viral load was 8.01 × 106 GC/g, 2.03 × 106 GC/mL, and 1.36 × 105 GC/mL in stool, sera, and urine, respectivelly. Of all patients, 88.57% had previous comorbidities, and 48.39% of them had detectable SARS-CoV-2 RNA in at least one type of clinical specimen evaluated by this study (stool, sera or urine). A higher viral load was observed in patients with more than two previous comorbidities and that were classified as severe or critical conditions. Samples with the highest viral loads were sequenced and characterized as B.1.1.33 variant.
Conclusion: We conclude that SARS-CoV-2 RNA is present in more than one type of clinical specimen during the infection, and that the most critical patients had detectable viral RNA in more than one clinical specimen at the same time point.
Keywords: B.1.1.33 variant; SARS-CoV-2 RNA; comorbidity; viral shedding; whole-genome sequencing.
© 2021 The Author(s).
Conflict of interest statement
None.
Figures

References
-
- W.H.O. WHO; 2021. WHO Health Emergency Dashboard.https://covid19.who.int/ 2021.
-
- W.H. Organization . World Health Organization; 25 January 2021. COVID-19 Clinical management: Living Guidance.https://apps.who.int/iris/bitstream/handle/10665/333912/WHO-2019-nCoV-Su... No. WHO/2, World Health Organization, https://app.magicapp.org/#/guideline/j1WBYn/rec/EalkPn, 2021. (accessed May 3, 2021)
-
- N.I. of NIH, Health. 2021. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines; pp. 1–354.https://www.covid19treatmentguidelines.nih.gov/ - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
