This is a preprint.
Gut microbiome dysbiosis during COVID-19 is associated with increased risk for bacteremia and microbial translocation
- PMID: 35262080
- PMCID: PMC8902880
- DOI: 10.1101/2021.07.15.452246
Gut microbiome dysbiosis during COVID-19 is associated with increased risk for bacteremia and microbial translocation
Update in
-
Gut microbiome dysbiosis in antibiotic-treated COVID-19 patients is associated with microbial translocation and bacteremia.Nat Commun. 2022 Nov 1;13(1):5926. doi: 10.1038/s41467-022-33395-6. Nat Commun. 2022. PMID: 36319618 Free PMC article.
Abstract
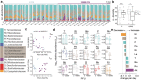
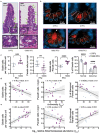
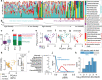
The microbial populations in the gut microbiome have recently been associated with COVID-19 disease severity. However, a causal impact of the gut microbiome on COVID-19 patient health has not been established. Here we provide evidence that gut microbiome dysbiosis is associated with translocation of bacteria into the blood during COVID-19, causing life-threatening secondary infections. Antibiotics and other treatments during COVID-19 can potentially confound microbiome associations. We therefore first demonstrate in a mouse model that SARS-CoV-2 infection can induce gut microbiome dysbiosis, which correlated with alterations to Paneth cells and goblet cells, and markers of barrier permeability. Comparison with stool samples collected from 96 COVID-19 patients at two different clinical sites also revealed substantial gut microbiome dysbiosis, paralleling our observations in the animal model. Specifically, we observed blooms of opportunistic pathogenic bacterial genera known to include antimicrobial-resistant species in hospitalized COVID-19 patients. Analysis of blood culture results testing for secondary microbial bloodstream infections with paired microbiome data obtained from these patients indicates that bacteria may translocate from the gut into the systemic circulation of COVID-19 patients. These results are consistent with a direct role for gut microbiome dysbiosis in enabling dangerous secondary infections during COVID-19.
Figures
References
Publication types
Grants and funding
- P30 CA016087/CA/NCI NIH HHS/United States
- R01 DK093668/DK/NIDDK NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- R21 AI139374/AI/NIAID NIH HHS/United States
- OT2 HL161847/HL/NHLBI NIH HHS/United States
- R01 AI140754/AI/NIAID NIH HHS/United States
- R01 AI130945/AI/NIAID NIH HHS/United States
- S10 OD021747/OD/NIH HHS/United States
- R01 AI121244/AI/NIAID NIH HHS/United States
- T32 AI100853/AI/NIAID NIH HHS/United States
- R01 AI143639/AI/NIAID NIH HHS/United States
- R01 HL123340/HL/NHLBI NIH HHS/United States
- DP2 AI164318/AI/NIAID NIH HHS/United States
- R01 DK124336/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous
