This is a preprint.
High Viral Specific Antibody Convalescent Plasma Effectively Neutralizes SARS-CoV-2 Variants of Concern
- PMID: 35262085
- PMCID: PMC8902868
- DOI: 10.1101/2022.03.01.22271662
High Viral Specific Antibody Convalescent Plasma Effectively Neutralizes SARS-CoV-2 Variants of Concern
Update in
-
Convalescent plasma with a high level of virus-specific antibody effectively neutralizes SARS-CoV-2 variants of concern.Blood Adv. 2022 Jun 28;6(12):3678-3683. doi: 10.1182/bloodadvances.2022007410. Blood Adv. 2022. PMID: 35443020 Free PMC article.
Abstract
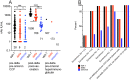
The ongoing evolution of SARS-Co-V2 variants to omicron severely limits available effective monoclonal antibody therapies. Effective drugs are also supply limited. Covid-19 convalescent plasma (CCP) qualified for high antibody levels effectively reduces immunocompetent outpatient hospitalization. The FDA currently allows outpatient CCP for the immunosuppressed. Viral specific antibody levels in CCP can range ten-to hundred-fold between donors unlike the uniform viral specific monoclonal antibody dosing. Limited data are available on the efficacy of polyclonal CCP to neutralize variants. We examined 108 pre-delta/pre-omicron donor units obtained before March 2021, 20 post-delta COVID-19/post-vaccination units and one pre-delta/pre-omicron hyperimmunoglobulin preparation for variant specific virus (vaccine-related isolate (WA-1), delta and omicron) neutralization correlated to Euroimmun S1 IgG antibody levels. We observed a 2-to 4-fold and 20-to 40-fold drop in virus neutralization from SARS-CoV-2 WA-1 to delta or omicron, respectively. CCP antibody levels in the upper 10% of the 108 donations as well as 100% of the post-delta COVID-19/post-vaccination units and the hyperimmunoglobulin effectively neutralized all three variants. High-titer CCP neutralizes SARS-CoV-2 variants despite no previous donor exposure to the variants.
Key points: All of the post-delta COVID-19/post vaccination convalescent plasma effectively neutralizes the omicron and delta variants.High-titer CCP and hyperimmunoglobulin neutralizes SARS-CoV-2 variants despite no previous donor exposure to the variants.
Conflict of interest statement
Conflict-of-interest disclosure
TG- paid consultant for Fresenius Kabi; GC and RM are on the Board of Innovative Transfusion Medicine; WB-Board of Blood Centers of America; AC- Scientific Advisory Board of Sabtherapeutics (cow-derived human immunoglobulins COVID-19 treatment and other infectious diseases) and Ortho Diagnostics Speakers Bureau; EB- member of the FDA Blood Products Advisory Committee. All other authors report no relevant disclosures.
Figures

References
-
- Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2021. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
