This is a preprint.
Multicenter analysis of neutrophil extracellular trap dysregulation in adult and pediatric COVID-19
- PMID: 35262093
- PMCID: PMC8902885
- DOI: 10.1101/2022.02.24.22271475
Multicenter analysis of neutrophil extracellular trap dysregulation in adult and pediatric COVID-19
Update in
-
Multicenter analysis of neutrophil extracellular trap dysregulation in adult and pediatric COVID-19.JCI Insight. 2022 Aug 22;7(16):e160332. doi: 10.1172/jci.insight.160332. JCI Insight. 2022. PMID: 35852866 Free PMC article.
Abstract
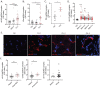
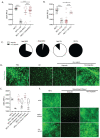
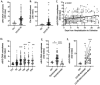
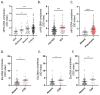
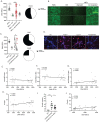
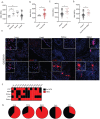
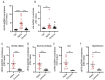
Dysregulation in neutrophil extracellular trap (NET) formation and degradation may play a role in the pathogenesis and severity of COVID-19; however, its role in the pediatric manifestations of this disease including MIS-C and chilblain-like lesions (CLL), otherwise known as "COVID toes", remains unclear. Studying multinational cohorts, we found that, in CLL, NETs were significantly increased in serum and skin. There was geographic variability in the prevalence of increased NETs in MIS-C, in association with disease severity. MIS-C and CLL serum samples displayed decreased NET degradation ability, in association with C1q and G-actin or anti-NET antibodies, respectively, but not with genetic variants of DNases. In adult COVID-19, persistent elevations in NETs post-disease diagnosis were detected but did not occur in asymptomatic infection. COVID-19-affected adults displayed significant prevalence of impaired NET degradation, in association with anti-DNase1L3, G-actin, and specific disease manifestations, but not with genetic variants of DNases. NETs were detected in many organs of adult patients who died from COVID-19 complications. Infection with the Omicron variant was associated with decreased levels of NETs when compared to other SARS-CoV-2 strains. These data support a role for NETs in the pathogenesis and severity of COVID-19 in pediatric and adult patients.
Summary: NET formation and degradation are dysregulated in pediatric and symptomatic adult patients with various complications of COVID-19, in association with disease severity. NET degradation impairments are multifactorial and associated with natural inhibitors of DNase 1, G-actin and anti-DNase1L3 and anti-NET antibodies. Infection with the Omicron variant is associated with decreased levels of NETs when compared to other SARS-CoV-2 strains.
Figures

References
-
- 2021. COVID research: a year of scientific milestones. Nature - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
