Disclosing Cyclic(Alkyl)(Amino)Carbenes as One-Electron Reductants: Synthesis of Acyclic(Amino)(Aryl)Carbene-Based Kekulé Diradicaloids
- PMID: 35262232
- PMCID: PMC9321839
- DOI: 10.1002/chem.202104567
Disclosing Cyclic(Alkyl)(Amino)Carbenes as One-Electron Reductants: Synthesis of Acyclic(Amino)(Aryl)Carbene-Based Kekulé Diradicaloids
Abstract
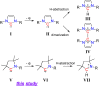
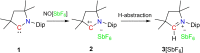
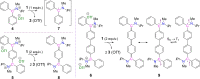
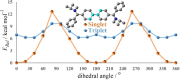
Herein, we disclose cyclic(alkyl)(amino)carbenes (CAACs) to be one-electron reductants under the formation of a transient radical cation as indicated by EPR spectroscopy. The disclosed CAAC reducing reactivity was used to synthesize acyclic(amino)(aryl)carbene-based Thiele and Chichibabin hydrocarbons, a new class of Kekulé diradicaloids. The results demonstrate CAACs to be potent organic reductants. Notably, the acyclic(amino)(aryl)carbene-based Chichibabin's hydrocarbon shows an appreciable population of the triplet state at room temperature, as evidenced by both variable-temperature NMR and EPR spectroscopy.
Keywords: EPR spectroscopy; carbene; electrochemistry; radical cation; reductant.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources

