"Reframing" dopamine signaling at the intersection of glial networks in the aged Parkinsonian brain as innate Nrf2/Wnt driver: Therapeutical implications
- PMID: 35262262
- PMCID: PMC9009237
- DOI: 10.1111/acel.13575
"Reframing" dopamine signaling at the intersection of glial networks in the aged Parkinsonian brain as innate Nrf2/Wnt driver: Therapeutical implications
Abstract
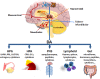
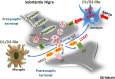
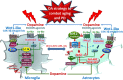
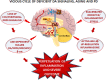
Dopamine (DA) signaling via G protein-coupled receptors is a multifunctional neurotransmitter and neuroendocrine-immune modulator. The DA nigrostriatal pathway, which controls the motor coordination, progressively degenerates in Parkinson's disease (PD), a most common neurodegenerative disorder (ND) characterized by a selective, age-dependent loss of substantia nigra pars compacta (SNpc) neurons, where DA itself is a primary source of oxidative stress and mitochondrial impairment, intersecting astrocyte and microglial inflammatory networks. Importantly, glia acts as a preferential neuroendocrine-immune DA target, in turn, counter-modulating inflammatory processes. With a major focus on DA intersection within the astrocyte-microglial inflammatory network in PD vulnerability, we herein first summarize the characteristics of DA signaling systems, the propensity of DA neurons to oxidative stress, and glial inflammatory triggers dictating the vulnerability to PD. Reciprocally, DA modulation of astrocytes and microglial reactivity, coupled to the synergic impact of gene-environment interactions, then constitute a further level of control regulating midbrain DA neuron (mDAn) survival/death. Not surprisingly, within this circuitry, DA converges to modulate nuclear factor erythroid 2-like 2 (Nrf2), the master regulator of cellular defense against oxidative stress and inflammation, and Wingless (Wnt)/β-catenin signaling, a key pathway for mDAn neurogenesis, neuroprotection, and immunomodulation, adding to the already complex "signaling puzzle," a novel actor in mDAn-glial regulatory machinery. Here, we propose an autoregulatory feedback system allowing DA to act as an endogenous Nrf2/Wnt innate modulator and trace the importance of DA receptor agonists applied to the clinic as immune modifiers.
Keywords: Nrf2/Wnt signaling; Parkinson's disease; dopamine signaling; glial-neuron crosstalk; inflammation; oxidative stress.
© 2022 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
We declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

